Online Submissions
Already have a Username/Password for Urology Herald?
Go to Login
Need a Username/Password?
Go to Registration
Registration and login are required to submit items online and to check the status of current submissions.
Start submissionAuthor Guidelines
General Information
It is strongly recommended to follow the AUTHORS GUIDELINES adopted in the journal "Urology Herald — Vestnik Urologii" and draw up considering the Recommendations for the Conduct, Reporting, Editing, and Publications of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors (ICMJE).
The journal accepts for publication original and discussion articles, reviews, clinical observations, lectures, historical essays on urology and outstanding researchers, reports on scientific and educational events. Special sections publish clinical guidelines on urological diseases and new medical technologies in the field of urology.
The journal accepts manuscripts from specialists and experts in various fields of urology, andrology, oncological urology, urological infections, neurourology, pediatric urology, urogynecology, and urological transplantology, graduate and doctoral students, applicants.
The journal recommends that authors use in the preparation of manuscripts and other scientific materials the following checklists developed by international healthcare organizations (EQUATOR, Enhancing the Quality and Transparency of Health Research)
- randomized trials – «CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials»;
- non-experimental (observational) studies - The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies»;
- systematic reviews and meta-analysis - «The PRISMA 2020 statement: An updated guideline for reporting systematic reviews»;
- clinical cases - «The CARE Guidelines: Consensus based Clinical Case Reporting Guideline Development»;
- qualitative research - «Standards for reporting qualitative research (SRQR): a synthesis of recommendations»;
- diagnostic / prognostic studies - «STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies»;
- animal pre-clinical studies - «The ARRIVE Guidelines 2.0: updated guidelines for reporting animal research».
Manuscripts and accompanying files must be uploaded through the electronic editorial system of the website https://www.urovest.ru. Authors are required to prepare the following files for submission of the manuscript:
1) main manuscript file in Microsoft Word format (*.doc / *.docx files);
2) illustrations in separate files (one *.zip / *.rar archive folder);
3) scanned manuscript submission form (*.pdf file);
4) scanned cover letter form (*.pdf file).
Before submitting an article for consideration, make sure that the files contain all the necessary information in accordance with the AUTHORS GUIDELINES adopted in the journal "Urology Herald — Vestnik Urologii".
It is necessary to use approved templates for the preparation of the manuscript and accompanying files:
Manuscript Submission Form template
We draw the attention of the authors - manuscripts that do not comply with the rules and requirements of the journal are not accepted for consideration!
Section I. Requirements for information about the manuscript and authors
1. The title page of the manuscript should contain the following information:
- type of manuscript / journal section (original article; discussion article; review; clinical case; current state-of-the-art; lecture; new medical technologies; guidelines; exchange of practical experience; “life-hacks” for a urologist; report on scientific and educational events; history of urology);
- the title of the manuscript (the maximum length — 20 words);
- names, middle names, and surnames of the authors styled as (Ivan I. Ivanov / Jonh P. Smith);
- the full official name of the institution affiliated with authors (without identifying departments and divisions); authors must indicate all institutions related to the study; if authors from different institutions, it is necessary to indicate the belonging of each author to a particular institution using a superscript numerical index - 1, 2;
- the full mailing address of the institution, indicating country, city, postal code and street / avenue / line, etc. (in italics).
Design example:
[ORIGINAL ARTICLE]
Radical cystectomy for bladder cancer: early and late postoperative complications
© Oleg N. Vasilyev 1, Vadim A. Perepechay 1, 2, Andrey V. Ryzhkin 1
1 Rostov State Medical University
Russian Federation, 344022, Rostov-on-Don, 29 Nakhichevanskiy Ln.
2 Rostov Clinical Hospital — Southern District Medical Center, Federal Medical and Biological Agency
Russian Federation, 344023, Rostov-on-Don, 34 Peshkova St.
2. Abstract and keywords
The abstract is the source of the main research data for the information systems and databases that index the journal. It is the abstract that is the hallmark of the publication. Based on the assessment of this section of the article, the editors of the journal decide on the advisability of submitting it for review. After the publication of the article, the majority of readers are limited to reading the abstract (according to some reports, up to 95%). The reader should be clear about the essence of the study, which will encourage him to read the full text of the article for detailed information.
The abstract must be submitted for all types of manuscripts. For original articles, the abstract must be MANDATORY structured and include basic sections reflecting the chronological order of the study: Introduction and Purpose of the study, Materials and methods, Results, Conclusion.
For reviews, clinical observations, and other types of publications, structured abstracts are preferred, but not required, while maintaining consistency.
The abstract should avoid introductory phrases and non-specific lengthy wording. It should not contain unmarked abbreviations, acronyms and symbols, terms introduced for the first time, references to literature sources, and illustrations. The volume of the abstract text is determined by the content of the manuscript (the amount of information, their scientific value and / or practical significance). Mentioning in abstract sections of various equipment / reagents / software must be accompanied by information about the manufacturer / developer in ROUND brackets stylized as - (“manufacturer”, city (and US state), country, year) [excluded from the total word count in the abstract]. The recommended length of the abstract is 200 - 300 words (no more than 300).
The abstract ends with keywords on the subject of the manuscript. It is desirable that the keywords complement the abstract and title of the article. It is strongly recommended to use the MeSH Headings service when compiling a list of keywords. The recommended number of keywords is 3 - 10 words (no more than 10).
The abbreviations / acronyms used in the text of the manuscript should be given below. It is not recommended to use abbreviations if they are mentioned in the text less than 5 times.
Design example:
Keywords: chronic bacterial prostatitis; diagnosis; lower urinary tract symptoms; microbiota
Abbreviations: chronic bacterial prostatitis (CBP); prostate cancer (PCa); urinary tract infections (UTIs)
3. Accompanying data and Ethical statement
- Funding sources and Acknowledgments
In this section, authors must indicate all sources of financial support (grant, foundation, commercial or government organization, company, individual, etc.) with a sequential indication of the organization / source of funding, grant / agreement number, date of approval of the grant / agreement, as well as acknowledgments to the researchers who participated in the preparation of the manuscript but are not the authors. Acknowledgments imply: recommendations for improving the study, providing space for research, departmental control, obtaining financial support, selected types of analysis, providing reagents / patient data (with their consent) / animals / other materials for research, etc.
Design example:
Financing. The study was not sponsored. OR The study was conducted with funding of the Russian Fund for Basic Research, grant No. 167524-917-085 signed 12 January 2022.
Acknowledgements. The authors are grateful to John P. Smith for assistance in the advanced statistical processing during the drafting of an article.
- Conflict of interest disclosure
In this section, authors are required to disclose any apparent or potential conflict of interest when submitting a manuscript. Concealment of potential and obvious conflicts of interest revealed by the editors may be the reason for the refusal to consider and publish the manuscript. If there is no conflict of interest, the authors should also indicate this.
Design example:
Conflict of interest. The author(s) declare(s) no conflict of interest. OR Ivan I. Ivanov declares that he is a (an) lecturer / ambassador / freelancer / employee of the …. indicate the native or English name of the non-governmental organization / institution / corporation / pharmaceutical company, etc., the date of approval of the current contract.
Pay attention! The existence of a conflict of interest (the financial interest of the author(s) or the assistance of any public or private organizations at certain stages of the study and / or compiling the manuscript) is not a reason for refusing to publish the manuscript. Contrastingly, this information with a reliable disclosure of a conflict of interest will indicate transparency of the authors and the credibility of the results, which will give the manuscript certain advantages at the stage of evaluation by reviewers and will arouse greater interest and trust of readers.
- Ethical statement
In this section, authors are required to provide information about the ethical approval of the study, indicating the official name of the ethics committee, protocol number and date of its signing (applicable to certain types of study).
Research with human participation must comply with the ethical principles of the Declaration of Helsinki, developed by the World Medical Association - "Ethical principles for conducting scientific medical research involving humans" (64th WMA General Assembly, Fortaleza, Brazil, October 2013) and the Order of the Ministry of Healthcare of the Russian Federation dated 01 April 2016 No. 200n "On approval of the rules of good clinical practice".
Design example:
Ethical statement. The study was approved by the Ethics Committee of the Rostov State Medical University (Protocol No. 14/22 signed 22 October 2021). AND / OR The study was designed according to the prescriptions of the Declaration of Helsinki (revised in 64th WMA General Assembly, Fortaleza, Brazil, October 2013).
Pay attention! If the trial is registered with the United States National Library of Medicine or the International Standard Randomized Controlled Trial Number Registry, please provide the relevant database, protocol number, and registration date after the Ethical statement.
- Patient Rights and Bioethics
Patients have the right to privacy, which cannot be disclosed without their consent. Personally identifiable information, including first and last names, hospital, and case history number, should not be published in the form of descriptions and / or photographs, unless it is of great scientific value or unless the patient (or his(her) parents / officials) provides consent to publication. In such a case, authors should inform patients of the likelihood that material identifying them will be available via the Internet after publication. However, in any case, before publishing a manuscript containing personal data, the authors must obtain a signed informed consent from the patient for the processing of personal data and report this in the appropriate section of the accompanying data.
Design example:
Informed consent. All patients signed informed consent to participate in the study and to process personal data. OR The patient's parents / officials signed an informed consent to participate in the study and to process personal data (if patients are under 18 years old / disabled). OR Patient signed an informed consent to process and publish personal data (for clinical case only).
When using laboratory animals in a study, it is necessary to indicate whether the study protocol complied with the Ethical principles and standards for conducting biomedical research involving animals: the European Convention for the Protection of Vertebrate Animals used for Research and other Scientific Purposes (ETS No. 123) and / or the Federation of European Associations for Science on Laboratory Animals (FELASA) and/or the International Council for the Science of Laboratory Animals (ICLAS) and/or national laws (indicate the country, number and date of signing of the law) for the protection and humane care of animals.
Design example:
Compliance with the rules of bioethics. The study was carried out in accordance with the Ethical standards for the treatment of animals adopted by the European Convention for the Protection of Vertebrate Animals for Research and Other Scientific Purposes (CETS 123), the Federation of European Associations for the Laboratory Animal Science (FELASA) and the International Council for the Laboratory Animal Science (ICLAS).
- Authors’ contribution
Conforming the ICMJE guidelines, only those persons who have made a significant contribution to the concept and design of the study or to the acquisition, analysis, and interpretation of data, actively participated in the drafting of the manuscript or made fundamental changes, participated in the revision or final approval of the version of the manuscript, and agree to accept responsibility for the manuscript content.
specify: study concept, study design development, literature review, data acquisition, data analysis, statistical data processing, experiment design and implementation, biological material processing, drafting the manuscript, software support, scientific editing, critical review, supervision, other (clarify), etc.
Partial or full funding, provision of laboratory materials and devices, technical editing of the manuscript, scientific advice, or general leadership of the research team does not justify inclusion into the research team. All members of the research team who do not meet the criteria for authorship, but who provided assistance in conducting the study and compiling the manuscript, can be listed with their consent in the "Acknowledgments" section.
Design example:
Authors' contribution: Ivan I. Ivanov – study concept, study design development, data analysis, drafting the manuscript; John Smith – literature review, drafting the manuscript, software support; Anna P. Pavlova, Victoria N. Jefferson — data acquisition, data analysis, statistical data processing; Maximillian P.J. Oakenfold — data analysis, critical review, scientific editing, supervision.
Pay attention! Please note that for original article, clinical case, review, systematic review, and meta-analysis, it is mandatory to include in the contributions of the authors such positions as: supervision, critical review, scientific editing, study concept, study design development.
- Corresponding author
The corresponding author (responsible for communication with the editors) is a member of the research team who is responsible for the completeness of the presentation of the research team and the coordination with it of all changes made to the manuscript, based on the results of its review, and editing.
The corresponding author assumes primary responsibility for the published data. He confirms that he is fully acquainted with the data presented in the manuscript and has made a significant contribution to the creation of the manuscript. He also confirms that he will not make any changes to the text and any data of the manuscript without the consent of ALL authors. The corresponding author confirms his responsibility for further communication with the editors, making the necessary corrections to the text and / or data of the manuscript befitting the comments and suggestions of the editors and / or reviewers accordant with ALL authors.
Pay attention! Please include the phone number of the corresponding author to contact the editors (not for public access).
Design example:
Corresponding author: John P. Smith; e-mail: example@example.ru
- Imprint
The reference to the article must be in the National Library of Medicine (NLM Style) format.
Design example:
For citation: Ivanov II, Smith J, Petrov PP, Pavlova AP, Jefferson VN, Oakenfold MPJ. Manuscript title. Vestn.Urol.Year;Vol.(Issue):p-р. (In Russ.). DOI: 10.21886/2308-6424-2000-00-0-00-00.
Section IV. Manuscripts’ Text Guide
General requirements for formatting the text of the manuscript
Manuscripts are accepted in Microsoft Word text format (file extensions: *.doc, *.docx). The text must be typed in Hevletica / Arial. Font size — 14 pt. Line spacing — 2.0 pt. Indents on each side of the page 2.0 cm. Paragraph indentation — 1.5. Width alignment. Disable transfers.
Highlighting in the text can be done ONLY in italics or bold letters, but NOT underlining. It is necessary to remove from the text all repeated spaces and extra line breaks (in automatic mode through the Microsoft Word service “find and replace”). All pages should be numbered.
Only admitted symbols and abbreviations should be used in the articles. It is necessary to provide a transcript at the first mention when using abbreviations in the text. All values should be presented in International System of Units (ISU).
The authors mentioned in the article must be given with initials. A link to them must be indicated in the references. Surnames and given names of authors should be presented in the original transcription.
- Designate References in BOX BRAKETS, separated by commas ONLY, g. [6, 7, 8].
- Designate Figures and Tables in PARENTHESIS at the first mention, g. (Fig. 1), (Table 5).
- Abbreviations should be introduced at the FIRST mention of the term in the text, and enclosed in PARENTHESIS, g. Prostate cancer (PCa). IMPORTANT! It is not recommended to enter abbreviations that are mentioned in the text less than 5 times, except for commonly used ones, e.g. MRI, TRUS, MS-CT, etc.
- Microorganisms in the text at the FIRST mention are indicated in detail, g. Escherichia coli, Herpes simplex virus type 1, further abbreviated as E. coli, HPV-1. Species of microorganisms are designated as spp., e.g. Peptococcus spp., Candida spp. Italics are MANDATORY.
- Genes and biochemical structures (enzymes, cytokines, specific proteins, etc.) in the text MUST be italicized (including abbreviations), e.g. bcl-5, urokinase, interleukin-6 (IL-6), haptoglobin, etc.
- When mentioning the names of foreign authors, after whom the methods, techniques of operations, scales, tools are named, it is MANDATORY to use the original spelling of the surname, and not transliteration, g. Foley catheter, Clavien-Dindo scale, ureteral reimplantation according to the Politano-Leadbetter method, Mann-Whitney U test, etc.
- When designating study groups, it is strictly NOT RECOMMENDED to use Roman numerals or superscript designations, g. I group, 1st group. RECOMMENDED designations — group 1, group 2 etc.
- When mentioning the names of researchers when describing their studies: if one author is mentioned, then USE the designation — Name (N.). Middle Name (M.). Surname, g. A. Smith, M.J. van Saac, I.I. Ivanov; if there is more than one author, USE the designation — Surname. Name (N.). Middle Name (M.). (Principal Investigator) et al. / et al., e.g. Smirnov A.N. et al., Muller M.N. et al. IMPORTANT! In all cases, after the designation of the author or research group, it is NECESSARY to indicate in parentheses the year of publication, e.g. Muller M.N. et al. (2019), I.I. Ivanov et al. (2017).
- USE ONLY en dashes (–) [Ctrl + Num-], g. 56 – 59%, 44.0 – 52.5. In text, USE ONLY an em dash (—) [Ctrl + Alt + Num-] as a punctuation mark, e.g. median — 6 cm. USE ONLY hyphen / numeric dash (-) [Num-] as a spelling mark, e.g. purple-red skin tone, one- and two-stage urethroplasty, Clavien-Dindo scale.
- When using special characters or symbols (<, >, /, ± и т.д.) that separate quantities or text, it is MANDATORY to separate the character or symbol with spaces on both sides, g. p > 0.05; 18.6 ± 2.3; laterally / medially. IMPORTANT! The forward slash (/) is NOT SEPARATED with spaces only in the case of units’ measure, e.g. mmol/l, mIU/ml.
- If necessary, the authors can allocate within the sections of the manuscript (introduction, materials and methods, etc.) subsections to focus on certain aspects of the study. Such subsections should start with a new paragraph and be italicized, g. Results of RP in patients of group 1, Results of RP in patients of group 2.
- Use of automatic numbering and bullets is ALLOWED.
- Highlighting text or changing the color of the font is UNACCEPABLE.
The editors reserve the right to adjust the articles submitted for publication. Authors should verify the manuscript's text on spelling and grammar correctness before submitting manuscript.
Original articles (original study, systematic review and meta-analysis)
The journal adheres to the IMRaD Structure format. The text of the original article must include the obligatory sections: Introduction and Objectives; Materials and methods; Results; Discussion; Conclusion.
Clinical and experimental studies must comply with accepted international principles of ethics and be carried out in accordance with international Good Clinical Practice (GCP) standards. When preparing the appropriate type of manuscript, it is STRICTLY recommended to follow the guidelines indicated in the "General Information" section of the Rules for authors — CONSORT 2010, STROBE, PRISMA 2020.
Formatting. The maximum size of a manuscript is 3500 words, excluding the list of references, tables, and figures. The number of words in the text can be found through the Word menu (“File” — “Document Properties” — “Statistics”). References — no more than 30. Tables — no more than 5. Figures — no more than 8.
Pay attention! Medicines must be referenced in International Non-proprietary Names (INN). The exception is original articles, in which it is permissible once only in the section "Materials & Methods" to indicate in brackets after the International Non-proprietary Names (INN) its trade name and manufacturer. If the article is devoted to a comparative analysis of the effectiveness of the original and generic drugs, then the international non-proprietary name is used throughout the text indicating the original drug or generic. Such articles are accepted for publication only if there is no conflict of interest, including hidden, not declared by the authors.
Introduction. The section contains a brief overview of the primary problem with references to the most significant publications (fundamental studies published over the past 5-7 years) and identifies unresolved issues, as well as the prerequisites for the study, and establishes a hypothesis to be tested. The recommended length of a section is <15% of the total word count in the manuscript.
Purpose of the study / Objective. Subsection containing a description of the primary research issue that required the study. The objective description should be as specific as possible. This subsection should be placed at the end of the Introduction. It is allowed to single out several subobjectives [1), 2)…], provided that the corresponding data presentation structure is preserved in the Results section. The recommended length of a section is <5% of the total word count in the manuscript.
Materials & methods. The section contains information about the locations where the study was conducted, as well as its duration; characteristics of the examined contingent of patients, criteria for inclusion and exclusion of patients in the study; study description (cohort, prospective, randomized, retrospective, etc.). It is advisable to describe the research method or methodology in detail if they differ in novelty (or they are interested in the context of this study). Methods published earlier must be accompanied by links to references; only the changes made to the method are described in detail. The research methods described should guarantee the reproducibility of the results. The equipment / reagents used in the study must be checked sequentially (the manufacturer and country are indicated in brackets). Drugs and chemicals used in the study should also be indicated in International Non-proprietary Names (INN), as well as doses and routes of administration.
Pay attention! The description of various equipment / reagents / software, etc., accompany in parentheses with information about the manufacturer / developer in the native language — (manufacturer, city (and US state), country, year), e.g. (Corporate Corp., CA, San Francisco, USA, 2017), (Ubermacht GmbH, Dortmund, Germany, 2010), («Nanomaterials» LLC, Yekaterinburg, Russian Federation, 2020).
- If the study so requires, then the Ethical statement must be indicated, i.e. by the decision of which Ethical Committee it was approved (its conclusion, protocol number, sign date, the official name of the Ethical Committee are quoted), the fact that the subjects signed the Informed Consent or the fact that the Rules of Bioethics (the rights of laboratory animals) were observed.
- At the end of the section, the Methods of Statistical Analysis are described in detail with the obligatory indication of the elements and methods of statistical analysis, statistical software, and version.
Results. The section should reflect the data obtained during research or experiment, literature search, and selection of publications (review article) that have undergone statistical analysis (if applicable). The results should be presented completely, clearly, and concisely, supported by figures, graphs, and tables, if necessary. FULL overlapping in the text of the data reflected in tables and graphs should be avoided, focusing on the description of the headmost points presented in the graphic materials. The recommended length of a section is 25-35% of the total word count in the manuscript.
Discussion. The section should summarize the main results, critically evaluate, and analyze the materials and methods used, compare the results with published data, and discuss the significance of the results. It is allowed to use tables, figures, and graphs in this section (to compare the received and known data). At the end of the section, the Limitations of this study should be reflected. The recommended length of a section is 25-35% of the total word count in the manuscript.
Conclusion. The section should briefly reflect the significance of the study, formulate the main conclusions, and identify prospects for research in this area. At the end of the section, 3 – 5 (or more) Keypoints should be formulated, based on the results obtained during the study, which have practical or fundamental theoretical significance. The recommended length of a section is 10-15% of the total word count in the manuscript.
Supplementary materials (optional). The section should contain links to external resources and cloud storage with a description containing accompanying materials for the manuscript or figures that exceed the established limit for a particular type of manuscript, electronic data sets, highly detailed drawings or animated graphics, applications for laptops or smartphones, videos, presentations, etc. This section is not included in the total word count of the manuscript.
Pay attention!! When including additional materials, the section Accompanying data before the Corresponding author should be added with the Supplementary materials column, in which it is necessary to indicate the type of additional materials (for example, “Video operation: Robotic nephroureterectomy on the left”) and accompany the column at the end with the phrase: The authors confirm the digital security of content hosted on external resources and cloud storages. Duration of the link from mm/dd/yyyy to mm/dd/yyyy.
Reviews
The purpose of the review is to highlight the accumulated scientific data, discuss various points of view and present the author's own view of the facts presented, but not to list and evaluate the current state-of-the-art.
Formatting. The maximum size of a manuscript is 5,000 words, excluding the list of references, tables, and figures. The number of words in the text can be found through the Word menu (“File” — “Document Properties” — “Statistics”). References — no more than 80. Tables — no more than 3. Figures — no more than 5.
It is recommended that the review be structured into relevant sections: Introduction & Objectives; Algorithm/Strategy of literary search (Materials & methods); Analysis of literature data (Results); Discussion; Conclusion. It may also contain the necessary graphic material (figures, graphs, tables) to facilitate the perception of the text. It is preferable that the compilation of the reviews be in-line with international guides for systematic literature search methods and international standards. The algorithm for searching and selecting articles in the Scopus, Web of Science, PubMed/MedLine, The Cochrane Library, eLIBRARY etc. databases should be specified in the Algorithm/Strategy of literary search (Materials & methods) section. The abstract must also contain this information. Review articles should include the word "review" in the Кeywords section.
The main issue of the problem addressed in the Review should be divided into thematic subsections of the Analysis of literature data. Discussion of various points of view and the presentation of the authors' own view of the facts presented can be reflected both separately in the Discussion section, or the main text of the manuscript.
At the end of the Conclusion section, 3 – 5 (or more) Keypoints should be formulated, based on the results obtained during the analysis of the literature data, which have practical or fundamental theoretical significance. The review should indicate all sources of primary information used. The references should contain, in addition to fundamental studies, publications mainly for the last 5 – 7 years (70 – 80%), links to highly cited sources. Links must be verified and widely visible.
Supplementary materials (optional). The section should contain links to external resources and cloud storage with a description containing accompanying materials for the manuscript or figures that exceed the established limit for a particular type of manuscript, electronic data sets, highly detailed drawings or animated graphics, applications for laptops or smartphones, videos, presentations, etc. This section is not included in the total word count of the manuscript.
Pay attention!! When including additional materials, the section Accompanying data before the Corresponding author should be added with the Supplementary materials column, in which it is necessary to indicate the type of additional materials (for example, “Video operation: Robotic nephroureterectomy on the left”) and accompany the column at the end with the phrase: The authors confirm the digital security of content hosted on external resources and cloud storages. Duration of the link from mm/dd/yyyy to mm/dd/yyyy.
Clinical cases
This type of manuscript is aimed at presenting and disclosing a clinical case with specific clinical course, diagnostic algorithm or treatment of common diseases or description of sporadic urological pathology. For clinical cases, it is MANDATORY to follow the structure of the article, including the Introduction; Patient information; Clinical presentation; Discussion; Conclusion.
Formatting. The maximum size of a manuscript is 2000 words, excluding the list of references, tables, and figures. The number of words in the text can be found through the Word menu (“File” — “Document Properties” — “Statistics”). References — no more than 10. Tables — no more than 3. Figures — no more than 5. To describe a clinical case, it is RECOMMENDED to use the recommendations indicated in the "General Information" section of the Rules for authors — CARE.
Introduction. The section should contain the rationale for the presentation of this case (its uniqueness, relevance, etc.) and a brief reference to the literature.
Patient information. The section should include de-identified information about the patient (gender, age, etc.), complaints, medical, family and disease history, history of relevant previous interventions with outcomes.
Clinical presentation. The section should reflect the timeline, clinical findings, diagnostic assessment, preliminary and final diagnosis, treatment interventions, information about the course of the disease, a description of possible complications, prospects for follow-up and outcomes and supplemented by illustrations for the clinical case.
Discussion. The section should contain a description of the strengths and limitations associated with this clinical case, comparison and analysis of the results of the examination and treatment with the data of the relevant medical literature on this case with references.
Conclusion. The section should summarize the features of this clinical case and the value of the experience gained. It is recommended that at the end of this section, the Patient's perspective on the treatment he received should be presented in one or two paragraphs.
Supplementary materials (optional). The section should contain links to external resources and cloud storage with a description containing accompanying materials for the manuscript or figures that exceed the established limit for a particular type of manuscript, electronic data sets, highly detailed drawings or animated graphics, applications for laptops or smartphones, videos, presentations, etc. This section is not included in the total word count of the manuscript.
Pay attention!! When including additional materials, the section Accompanying data before the Corresponding author should be added with the Supplementary materials column, in which it is necessary to indicate the type of additional materials (for example, “Video operation: Robotic nephroureterectomy on the left”) and accompany the column at the end with the phrase: The authors confirm the digital security of content hosted on external resources and cloud storages. Duration of the link from mm/dd/yyyy to mm/dd/yyyy.
For other types of manuscripts (historical essays, lectures, comments, reports on scientific and practical events, etc.), it is OPTIONAL to follow the IMRaD structure of the manuscript. However, a certain structure of the text and clarity of presentation of the material must be observed.
Formatting. The maximum size of a manuscript is 2000 words and 5000 words ONLY for lectures, excluding the list of references, tables, and figures. The number of words in the text can be found through the Word menu (“File” — “Document Properties” — “Statistics”). References — no more than 20 and 50 ONLY for lectures. Tables — no more than 2. Figures — no more than 3.
Supplementary materials (optional). The section should contain links to external resources and cloud storage with a description containing accompanying materials for the manuscript or figures that exceed the established limit for a particular type of manuscript, electronic data sets, highly detailed drawings or animated graphics, applications for laptops or smartphones, videos, presentations, etc. This section is not included in the total word count of the manuscript.
Pay attention!! When including additional materials, the section Accompanying data before the Corresponding author should be added with the Supplementary materials column, in which it is necessary to indicate the type of additional materials (for example, “Video operation: Robotic nephroureterectomy on the left”) and accompany the column at the end with the phrase: The authors confirm the digital security of content hosted on external resources and cloud storages. Duration of the link from mm/dd/yyyy to mm/dd/yyyy.
Section III. Requirements for illustrations
The illustrations are photographs, drawings, diagrams, graphs, charts, and tables. Illustration files must be of high quality for correct display in the online version of the journal. The authors must provide the editors with permission from the copyright holder to publish any illustration in this journal if it has previously been published in other editions or journals. The number of illustrations should correspond to the amount of information provided. The editors can return the article to the authors for revision in order to reduce the amount of illustrations if the manuscript contains an excess of them, thereby increasing its volume.
The illustrations are placed optionally in the sequence as mentioned in the text or grouped together at the end of the manuscript. If necessary, illustrations can be sent as separate files through the editorial submission manager — Step 4. UPLOAD ADDITIONAL FILES.
- Figures and graphs
All figures and graphs (diagrams, graphs, flowcharts, drawings, drawings, photographs, screenshots, etc.) are referred to as drawings, arranged sequentially and have a caption.
Each figure should be numbered with Arabic numerals as mentioned in the text. If there is only one figure in the article, then it is not numbered. If the figure consists of several parts, it is necessary to designate each part in LATIN uppercase - A, B, C, D, etc. References to the figures are made in the following way: “Figure 1 shows that ...” or “... demonstrated that ... (Fig. 1).”.
All figures must be provided with a caption. The figure caption includes the number of the figure in the text and its title. The figure number is separated from the title by a dot. A dot at the end of the caption is NOT PUT. Font size — 12 pt. Aligned to the left. The figure caption should be separated from the subsequent text element of the manuscript by an indent. The presence of abbreviations in the figure caption is NOT ALLOWED (except for generally accepted, e.g. MRI, CT etc.). All abbreviations and acronyms, designations of arrows, curves, letters, numbers, etc. used in the figure (on-picture) must be explained in the caption. Picturesque comments are allowed, font size — not less than 8 pt. The captions for microphotographs indicate the staining method and magnification.
Photos, screenshots, and other figures must be uploaded separately in a special section of the editorial submission manager (Step 4. UPLOAD ADDITIONAL FILES) as *.jpeg, *.png files (*.doc and *.docx — in case, if there are pictorial comments).
The figures or graphs used must be of sufficient quality, resolution of at least 300 x 300 dpi (linear graphics — at least 1000 x 1000 dpi). Color profile — RGB / sRGB / DCI-P3. The maximum file size is 5 Mb. Figure files must be given a file-name corresponding to the figure number in the text (for example: Fig. 1, Fig. 2A, Fig. 2B, etc.). All figure files are recommended to be combined into one *.zip or *.rar archive folder before sending.
To create algorithms and flow-charts, only standard shapes from the menu section "INSERT" — "SHAPES" should be used. SmartArt graphics are excluded.
The graph is created using the Microsoft Word application programs (menu sections "INSERT" — "SHAPES"), Microsoft Excel, GraphPad, Statistica, etc. If the graph is created in Microsoft Word / Excel, then it is preferable to design the graph in accordance with the template, presented below. The original Microsoft Excel file containing the graphs should be attached if editing is required.
Pay attention! It is preferable to use 2D graphics. Only if it is impossible to represent data in 2D, 3D graphics are acceptable. Color palette — avoid using bright colors; accents of blue, cyan, turquoise, gray, aquamarine are preferred. The chart must MANDATORY contain the data itself, the title of the axis, units of measurement (ONLY on the axis), the legend, the data labels. The font size of all text elements in the graph is 11 pt. The description of some text elements can be presented in the caption. When creating charts using specialized software (NOT MICROSOFT OFFICE software), compliance with the above requirements for formatting and styling is also MANDATORY.
Pay attention! INSERT GRAPHS FROM MICROSOFT EXCEL IN PICTURE FORMAT IS EXCLUDED!! INSERT IS CARRIED OUT IN THE FOLLOWING SEQUENCE OF USING THE MENU SECTIONS: "HOME" ⇒ "INSERT" ⇒ "KEEP INITIAL FORMATTING AND LINK DATA". When creating graphs in other application programs, compliance with the above formatting and styling requirements is also MANDATORY.
Design example
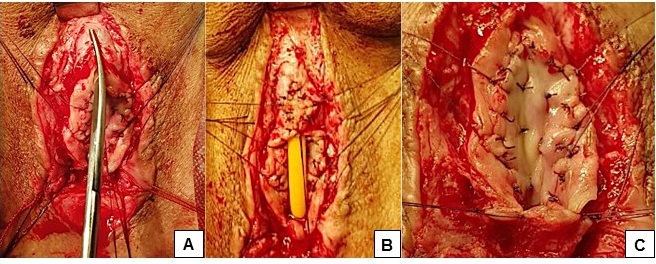
Figure 1. Techniques for suturing the ventral urethral semicircle after dorsal inlay augmentation urethroplasty: A — elongation of the initial urethrotomy; B — fixation of the diamond-shaped graft in the distal urethrotomy part; C — general view of the urethral bulb after applying and tying mattress sutures in the proximal urethrotomy part
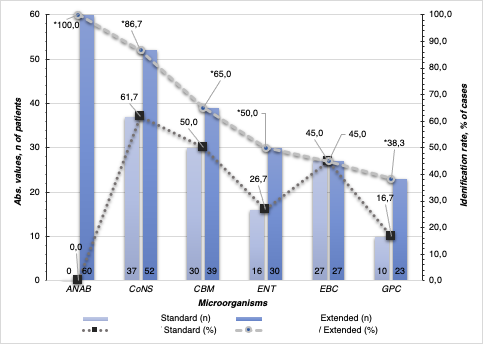
Figure 2. Results of standard and extended bacteriological studies for post-massage urine in patients (n = 60) from two groups (ANAB — Anaerobes, CoNS — Coagulase-negative Staphylococci; CBM — Corynebacterium spp., ENT — Enterococcus spp., EBC — Enterobacteriaceae, GPC - Gram + cocci); * – p<0.05 significance of differences between the standard / extended methods
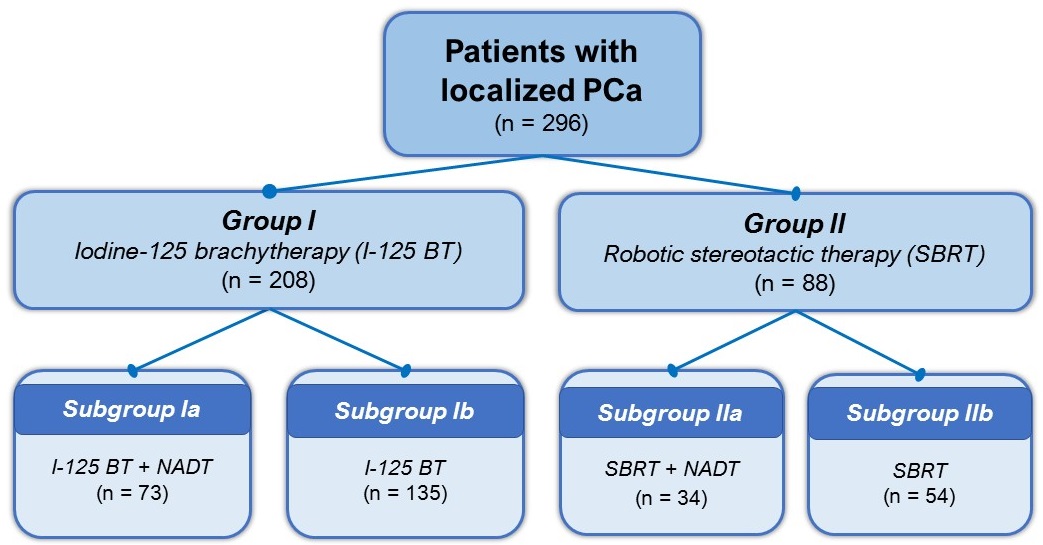
Figure 3. Study groups and subgroups (I-125 BT — Iodine-125 brachytherapy; SBRT — Robotic stereotactic therapy; NADT — Neoadjuvant androgen-deprivation therapy)
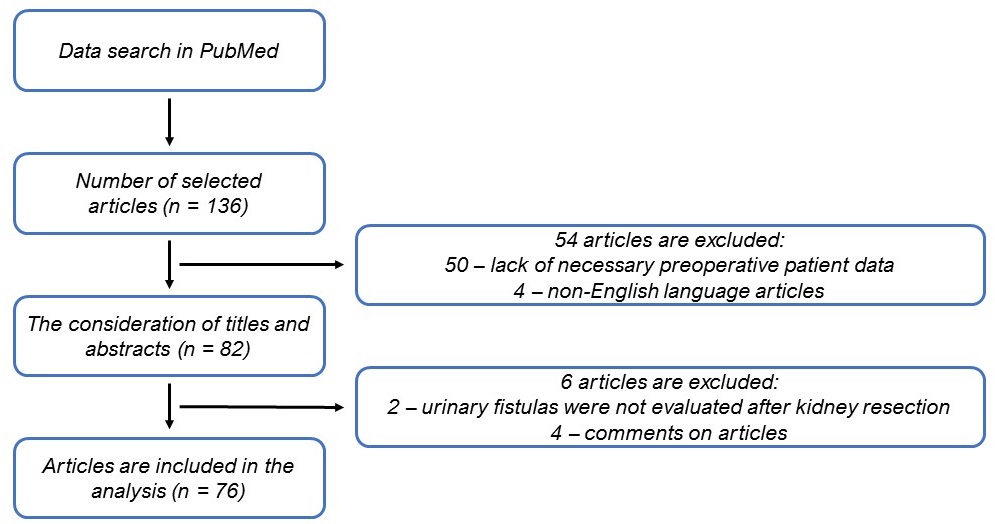
Figure 4. Selection algorithm for non-systematic review
- Tables
All tables should be informative, have a title, body, and note (if applicable). The data in the tables should complement and not repeat the data presented in the text and figures and vice versa.
Tables should be placed directly in the text of the manuscript as they are mentioned. Each table should be numbered consecutively in Arabic numerals in the text. If there is only one table in the manuscript, then it is not numbered.
References to tables are made as follows: “Table 1 indicates that ...” or “It is indicated that ... (Table 1).”
Pay attention! Tables must be editable. Scanned tables or tables as figures are NOT ALLOWED!
Tables should be created using Microsoft Word (menu sections "INSERT" ⇒ "TABLE"). Font size: 12 pt — table heading, 11 pt — table body, including column headings (column headings in bold), 10 pt — notes. Line spacing — 1.0 pt. Alignment: leftmost column, notes left, body centered.
The table heading includes the table number in the text and its title. The table number is separated from the title by a dot. The table heading is placed BEFORE the table. Do NOT put a dot after the table header. Aligned to the left. The heading should be separated from the previous element of the manuscript, line spacing — 12 pt.
The headings of the columns of the table must correspond to their content. The figures in the tables should correspond to the figures in the text and should MANDATORY have statistical processing designations (if applicable). Units of measurement (%, mmol/L, mIU/L, etc.) are allowed ONLY in the leftmost column or column heading and must be separated from the designation by a comma or parentheses.
Pay attention! When creating a column that reflects confidence values (p) between indicators, reduce the numerical representation to 3 decimal places. If there are confidence values (p) between the indicators that were determined using different statistical methods, indicate in the notes the corresponding methods using asterisks (*, **, ***).
Abbreviations and acronyms in tables are NOT ALLOWED (apart from generally accepted ones and in case of undeniable necessity). All abbreviations and acronyms used in a table, including its title, should be spelled out and explained in the notes to the table. The statistical methods used for the analysis and the corresponding confidence values (p) should also be indicated in the notes to the tables.
It is acceptable to create breaks in massive tables with a significant number of rows. In such cases, the table heading should be duplicated on a new page with an indication in parentheses (continuation). Horizontal arrangement of massive tables with a significant number of columns is also acceptable.
If tables are larger than A4 sheet, they must be submitted in a separate *.doc, *.docx, *.rtf format file through the editorial submission system — Step 4. UPLOAD ADDITIONAL FILES.
Design example:
Table 1. Patient demographics
| Characteristic | Value |
| Number of patients | 15 |
| Age, yrs (M + SD) | 57,87 ± 4,36 |
| Preoperative total PSA level, ng/ml (Me [LQ; UQ]) | 6,2 [5,1; 7,5] |
| IIEF, mean (M + SD) | 22,14 ± 3,21 |
| Parameters of penile hemodynamics measured with dynamic duplex ultrasound: | |
| PSV, mean cm/s (M + SD) | 38,0 ± 9,20 |
| EDV, mean cm/s (M + SD) | 7,11 ± 3,78 |
| RI mean (M + SD) | 0,81 ± 0,13 |
Notes: 1) M — mean; SD — standard deviation; Ме — median; LQ — 25% percentile; UQ — 75% percentile. 2) PSA — prostate specific antigen; IIEF — international index oferectile function; PSV — peak systolic velocity; EDV — end-diastolic velocity; RI — resistive index. | |
Section VI. References guidelines
The journal uses the Vancouver Reference Style. It implies a reference to the source in square brackets and the subsequent enumeration of sources in the references with sequence that it is mentioned in the article`s text (NOT in alphabetical sequence).
Formatting. Font size — 11 pt. Spacing — 1.0 pt. Left alignment.
Each scientific fact should be accompanied by a separate reference to the source [1]. If several scientific facts are mentioned in one sentence, then a link is made after each fact of citation (not at the end of the sentence). References are given in chronological order if there are a significant number of them [2, 5-7]. References to articles are not allowed if that are not in the reference list. On the contrary, all sources cited in the text should be included in the references list.
References should include only peer-reviewed sources (articles from scientific journals and monographs) referred to in the article`s text. It is undesirable to include thesis abstracts or thesis itself, textbooks, teaching aids, state standard specifications, websites and blogs information, statistical reports, articles in socio-political newspapers in the references. If it is necessary to refer to such information, it is preferable to put information about such a source in a footnote. References to unpublished manuscripts are not allowed.
The number of references is limited by the type of manuscript.
Footnotes. Footnotes should be numbered in Arabic numerals and it should be placed page by page. Footnotes may include: links to anonymous sources on the Internet, links to textbooks, manuals, state standard specifications, statistical reports, articles in political newspapers and journals, abstracts, dissertations (if it is not possible to cite articles published based on the results of a thesis research), comments by the author.
References should be presented not only in the original language, but also in English, considering the requirements of the International Citation Systems and Databases such as Scopus and Web of Science.
References are issued in the double-column table form:
- The first column indicates the source number in the order of its mention in the article`s text.
- The second column shows the references to the sources in the original language in accordance with the format approved by the International Committee of Medical Journal Editors — (MEDLINE/PubMed) [AMA 11th Reference Guide]. When citing, carefully follow the rules of international punctuation. Authors can view the full rules and examples for a reference list at [AMA 11th Reference Guide]
All references to journal publications should contain DOI if available (Digital Object Identifier, unique digital identifier of the article in CrossRef system). Authors can check for a DOI article at http://search.crossref.org or https://www.citethisforme.com. Authors can indicate PMID (unique article code in PubMed) in case of DOI is not available.
Design example:
- Journal article. Unnikrishnan R, Almassi N, Fareed K. Benign prostatic hyperplasia: evaluation and medical management in primary care. Cleve Clin J Med. 2017;84:53-64. DOI: 10.3949/ccjm.84a.16008
- Books (authors). Albala DM, Morey AF, Gomella LG, Stein JP, Reynard J, Brewster S, Biers S. Oxford American handbook of urology. New York: Oxford University Press Inc.; 2011.
- Books (ed.) For the whole book: Stolzenburg J-U, Türk IA, Liatsikos EN, eds. Laparoscopic and Robot-Assisted Surgery in Urology. Switzerland: Springer; 2011. On part of the book: Lewinsohn P. Depression in adolescents. In: Gotlib IH, Hammen CL, eds. Handbook of Depression. New York, NY: Guilford Press; 2002:541-553.
- Conference proceedings (citing is allowed if there is open access on the Internet only). Chu H, Rosenthal M. Search engines for the World Wide Web: a comparative study and evaluation methodology. Paper presented at: American Society for Information Science 1996 Annual Conference; October 19-24, 1996; Baltimore, MD. Available at: http://www.asis.org/annual-96/electronicproceeding.... Accessed February 26, 2004.
- Theses and dissertation abstracts (citing is allowed if there is open access on the Internet only). Fenster SD. Cloning and Characterization of Piccolo, a Novel Component of the Presynaptic Cytoskeletal Matrix [dissertation]. Birmingham: University of Alabama; 2000
- Patent (citing is allowed if there is open access on the Internet only). Rabiner RA, Hare BA, inventors; OmniSonics Medical Technologies Inc, assignee. Apparatus for removing plaque from blood vessels using ultrasonic energy. US patent 6,866,670. March 15, 2005.
- Web content (online). Department of Health & Human Services. Anaphylaxis. Better Health Channel. Updated August, 2014. Accessed August 31, 2020. Available at: https://www.betterhealth.vic.gov.au/health/ConditionsAndTreatments/anaphylaxis
The authors are fully responsible for the correctness of the data presented in reference list.
Section VI. Information about the authors
The last article page should contain:
- full name;
- academic degree, academic rank; position held, place of work (official name of the institution);
- city, country
- personal international identifier - ORCID;
- e-mail.
Pay attention!! It is MANDATORY to specify an ORCID identifier for all authors. In the absence of an ORCID number, it must be obtained by registering at https://orcid.org/.
Design example:
Information about the author(s) |
Oleg N. Vasilyev — M.D., Dr.Sc.(Med); Assist. Prof., Dept of Urology and Human Reproductive Health (with the Pediatric Urology and Andrology Course), Rostov State Medical University; Head, Urology Division, Rostov State Medical University Сlinic Rostov-on-Don, Russian Federation e-mail: vasilyev_on@mail.ru |
Jonh P. Smith — M.D., Ph.D., Prof.; Urologist, Dept. of Urology, Stokes Centre for Urology, The Royal Murray County Hospital NHS Foundation Trust Guildfolt, Murray, The United Kingdom https://orcid.org/0000-0002-0003-X420 e-mail: example@gmail.com
|
Nikolay N. Maitz — Dr.med., Prof.; Pediatric Urologist and Andrologist, Dept. for Urology, University Center Hamburg-Eppendorf Hamburg, Germany https://orcid.org/0000-0003-0004-5723 e-mail: example@outlook.com |
Section VII. Online form filling
Authors need to enter all article metadata in detail for its successful indexing in domestic and international databases when submitting a manuscript to the Editors through an online form:
- Authors. ATTENTION! Full names of Authors should be filled in English only. It is necessary to fill in the personal data of all Authors completely. E-mail of the Corresponding author will be published for communication with the Authors team in the article text (e-mail will be available to Internet users and subscribers of the journal print version in open access).
- Article title.
- Abstract. It must completely match the text in the manuscript file.
- Keywords. Authors must specify keywords (from 3 to 10) that contribute to indexing articles in search engines. Use the US National Medical Library thesaurus (Medical Subject Headings, MeSH) to select keywords.
- Language. Authors must indicate the language in which the manuscript full text is written. It is necessary to indicate double indexation by language, in the case when the author publishes an article in two languages. For example: [ru; en]
- References. Authors must draw up links in accordance with the "Reference guide" (see section VI).
- Extra data. Authors can send additional information to the Editors in the form of separate files (immediately after downloading the main manuscript file). Additional files include supporting documents, image files, source data (if the Authors want to submit their editions for review or at the request of Reviewers), video and audio files, which should be published together with the article in a journal online version. A description of each additional file should be entered before sending. Authors need to give the file the appropriate name if the information from the additional file should be published in the article text (thus, the description of the image file should contain a numbered signature, for example: Figure 3. Macroscopic view of the removed cyst).
- Completion of article submission. Authors need to check the list of sent files and complete the process of sending the article after downloading all the additional materials. The Editors will send a notification to the email address of the Corresponding author about the receipt of the article after completion of the sending procedure (within 7 days) (the absence of a letter is a confirmation that the Editors have not received the manuscript). Authors can contact the Editors at any time as well as track the stages of processing and reviewing their manuscript through a personal account on this site.
Section VIII. Article Publishing Procedure / Interaction between the Editorial board and the Authors
The journal Editors provide feedback with the Corresponding author by default. However, letters can be sent to all Authors (if they wish) whose e-mail was indicated.
Context editor carries out a preliminary check for compliance with the formal requirements of all incoming articles. Article can be returned to the author (s) for revision with a request to eliminate errors or add missing data at this stage. In addition, the Editor-in-chief and executive secretary because of its inconsistency with the objectives of the journal, lack of originality, and low scientific value, may reject the article.
Editor-in-chief decides on the choice of one or another Reviewer for the examination of the article after a preliminary check only. A notification is sent to the author that the article is under review.
Editor-in-chief may involve several specialists in the review process in controversial cases.
The journal Editors send a reasoned notice to the Corresponding author when deciding whether to refuse to publish the article.
The comments of the Reviewer are transmitted to the Corresponding author when deciding on the revision of the article. The Authors are given a period of 30 days to eliminate comments. The article is removed from the publication queue in case the Authors have not notified the Editors of the relevant changes during this period.
The final version of the article (accepted for publication) is sent to the Corresponding author. He is obliged to check the layout of the article, as well as to make sure that all the co-Authors saw and approved it. A response is expected from the Authors within 7 days. The layout of the article is considered "approved" in the absence of feedback from the Authors.
Article is transferred to the Context editor to prepare for publication in the event of a positive opinion of the Reviewer. The journal Editors inform the Corresponding author about this and indicate the date of article publication.
Average time from submission of a manuscript to publication: 120 days
Section IX. Procedure for reviewing editorial / Reviewer decisions
If the author does not agree with the opinion of the Reviewer and / or Editor or with individual comments, he can challenge the decision.
For this, the author needs:
- correct the manuscript of the article according to the justified comments of Reviewers and Editors;
- state your position on this issue clearly.
The journal Editors facilitate the re-submission of manuscripts that could potentially be accepted but were rejected due to the need for significant changes or the collection of additional data. The Editors are ready to explain in detail what needs to be corrected in the manuscript in order to it to be accepted for publication.
Section X. Detection of data plagiarism, fabrication or falsification: editorial actions
The Editors are guided by the rules of the Committee on Publication Ethics (COPE) in case of detection of unfair behavior by the author, detection of plagiarism, fabrication or falsification of data.
«Unfair behavior». By this term, the journal Editors understands any actions of a scientist, including improper handling of objects of study or intentional manipulation of scientific information (in which this information ceases to reflect the reality of the observed phenomena in the study) as well as the behavior of a scientist that does not meet accepted ethical and scientific standards.
The journal Editors does not attribute honest errors or honest differences in the design, conduct, interpretation or evaluation of research methods or results that are not associated with the scientific process as «unfair behavior».
Section XI. Bug fixes and article retraction
If errors are found in the article text that affect its perception, but do not distort the stated research results, they can be corrected by replacing the PDF-file of the article and indicating an error in the article file itself and on the article page on the journal website.
If errors are found in the article text that distort the research results, or in the case of plagiarism, detection of dishonest behavior of the author (or Authors) associated with falsification and / or fabrication of data, the article can be retracted (see section Journal Policy → Article Retraction).
If you have any questions, please contact the editors of the journal:
Anna V. Ilyash, e-mail: annailyash@yandex.ru
Ruslan S. Ismailov, e-mail: dr.ruslan.ismailov@gmail.com
Date of revision: February 25, 2022
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.
Submitted article has not been previously published and has not been submitted for review and publication in another journal (or the author provided an explanation of this incident in the Comments for Editors)
File of the submitted article is presented in Microsoft Word format and It has the file extension * .doc / * .docx
Complete Internet addresses (URLs) are provided for links (where it is possible)
The text is in Hevletica / Arial font. Size - 14 pt. Line spacing - 2.0 pt. Indents on each side of the page 2.0 cm. Paragraph indentation - 1.5. Width alignment. Transfers are disabled. Emphasis is in italics, not underlining (except for Internet addresses). All illustrations (figures, graphs and tables) are located at the end of the document and are duplicated in a separate file.
Article text corresponds with the stylistic and reference requirements described in the «Rules for Authors» sections, which are located on the page «About the Journal»
Potential and obvious conflicts of interest related to the manuscript are disclosed
Authors should indicate their own Personal International Identification Number (ORCID)
If you submit an article to the peer-reviewed journal`s section, you must comply with the requirements of the document “Providing blind peer-review”
Copyright Notice
Authors who publish articles in the «Vestnik Urologii» journal agree with the follow terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (The Effect of Open Access).
Privacy Statement
Specified when registering the names, e-mail, telephone numbers, information about job placement and other personal data will be used solely for technical purposes of a contact with the Author or Reviewers (Editors) when preparing the article for publication. Private data will not be shared with other individuals and organizations.











































