Scroll to:
Combination therapy for benign prostate hyperplasia-related urinary symptoms
https://doi.org/10.21886/2308-6424-2022-10-1-84-95
Abstract
Introduction. Non-neurogenic lower urinary tract symptoms (LUTS) are a serious polyetiologic problem in the male population. The side effects of the medication agents used to treat LUTS significantly reduce treatment compliance. According to the literature data, the frequency of refusal for the proposed treatment during the year varies from 20 to 80%. Several studies have shown the benefits of herbal medicine for LUTS concerning the fewer side effects and increased adherence to treatment. However, to obtain a high-level recommendation base, clinical trials are required.
Purpose of the study. To evaluate the effectiveness of Gardaprost® in LUTS combination therapy.
Materials and methods. The study included 57 men aged 60 – 70 years with diagnosed medium- or large-volume benign prostatic hyperplasia (BPH) and moderate-to-severe LUTS according to I-PSS, morphologically excluded prostate cancer, without urinary infection signs. The patients were randomized into two follow-up groups. Tamsulosin 0.4 mg q.d. was prescribed to patients in the control group. Patients of the main group received Gardaprost® 0.4 mg q.d. in addition to Tamsulosin. The follow-up period in both groups was one-year. The statistical analysis includes data from 56 men. To evaluate therapy at the screening visit and on days 180 and 360, I-PSS, urination diary, IIEF-5, urinalysis, prostate-specific antigen, uroflowmetry, ultrasound were analyzed. Paired t-test and one-way ANOVA test were used to determine intergroup differences in normally distributed variables. For variables with a distribution other than normal, Friedman's two-way ANOVA for related samples was used. Events with a probability greater than 95% were considered statistically significant.
Results. At the time of inclusion in the study, the groups were comparable concerning the control parameters. In the main group, there was a more pronounced positive dynamics in the I-PSS score, maximum urine flow rate, and post-void residual urine volume, which corresponded to 7.9 ± 2.1 points, 18.0 ± 7.3 ml/sec, 23.6 ± 13.6 ml vs 19.7 ± 7.2 points, 10 ± 3.5 ml/sec, 65.9 ± 33.2 ml in the main and control groups, respectively (p < 0.001). Additionally, in the main observation group, a decrease in prostate volume was recorded by 18.8% (p < 0.001) was recorded.
Conclusion. We have obtained encouraging long-term results from the use of Gardaprost® in combination therapy of moderate-to-severe LUTS caused by medium- and large-volume BPH.
Keywords
For citations:
Shkodkin S.V., Pokrovskiy M.V., Krasnyak S.S., Polishchuk A.V., Chirkov S.V., Churikova O.V., Kravtsova N.A. Combination therapy for benign prostate hyperplasia-related urinary symptoms. Urology Herald. 2022;10(1):84-95. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-84-95
Introduction
Lower urinary tract symptoms (LUTS) are considered a serious problem in both female and male populations and tend to progress with age [1]. Although LUTS is a polyetiological problem, in the male population, it is primarily caused by benign prostatic hyperplasia (BPH). LUTS associated with BPH become a significant social problem in older people and for the healthcare system, increasing the incidence rate of hospitalizations, the duration of incapacity to work and have disabilities, and outpatient costs [1][2][3]. Currently, the main variant of treatment for patients with BPH is pharmacotherapy [1][2]. The range of pharmacological means recommended for this therapy provides a personified approach to the treatment of various LUTS as monotherapy and in combination with other pharmacological agents [2][4]. However, some side effects of pharmacological agents should be considered that are summarized at the indication of combined therapy. This significantly reduces compliance with LUTS therapy. According to the available data, the rate of refusal of the prescribed therapy varies from 20 to 80%. The application of alpha-1-adrenoblockers, 5-alpha reductase inhibitors, M-cholinolytics, and phosphodiesterase-5 inhibitors is associated with a high risk of cardiovascular, gastrointestinal, cognitive, and ejaculatory disorders and erectile dysfunction [5]. The Guidelines of the International Gerontological Society recommend finasteride and trospium chloride as safe pharmacological agents for this category of patients. Besides, some researchers believe that polypragmasy is unacceptable for patients older than 60 years old, which debates the safety of combined therapy for LUTS in this age group of patients [3][6]. Some studies showed such advantages of herbal therapy for LUTS as fewer side effects and increased adherence to therapy. The possibilities of herbal therapy for LUTS have been long discussed in clinical recommendations of the leading urological societies [7][8][9]. However, to obtain a high-level recommendation base, clinical trials are required.
The study aimed to evaluate the effectiveness of Gardaprost® in LUTS combination therapy.
Materials and Methods
The study included 57 men with diagnosed BPH who signed informed consent for participation in the study and met the study entry criteria. The study entry criteria included age 60 –70 years old, 50 – 100 cm3 prostate volume, post-void residual urine > 200 ml, Qmax 5 – 15 ml/s (uroflowgram), intravesical prostate protrusion > 10 mm, IPSS 15 – 25 score; prostate-specific antigen (PSA) 4–10 ng/ml; patients with morphologically excluded prostate cancer, lack of inflammatory changes in urine test, and negative urine culture test. The criteria for non-inclusion in the study during the first visit were the following: previous surgical interventions in pelvic organs, thermotherapy, pelvic organs radiotherapy, bladder neck sclerosis or urethral stricture in medical history, complicated course of BPH (including stones and bladder diverticula, recurrent urinary infection, chronic bacterial prostatitis, upper urinary tract disorders), a requirement in surgical or any other urgent treatment for intercurrent diseases to prevent disease progression and health deterioration, simultaneous intake of 5-alpha-reductase inhibitors, chronic alcohol abuse, drug addiction, or psychic diseases, concomitant diseases at the stage of decompensation that can affect the study, hypersensitivity to any of the components of the studied and control drug, participation in other clinical studies within the last month or at the moment of inclusion to the study. After inclusion in the study, the patients were randomized into two groups. Patients in the control group received original OD tamsulosin 0.4 mg (1 capsule) OD to treat BPH-associated LUTS. The patients of the main group received tamsulosin and Gardaprost® 1 capsule (400 mg) OD regularly. The duration of the observation in both groups was 360 days. After treatment of outliers and extreme values, 56 men were included in the statistical analysis (30 patients in the main group and 26 patients in the control group).
The endpoints on 180 and 380 days were used to evaluate the expression of the symptoms based on the validated score surveys: severity of LUTS by IPSS (International Prostatic Symptom Score) and the diary of micturition filled in for 3 days. The quality of erectile function was assessed by the International Index of Erectile Function (IIEF-5). The dynamic of the therapeutic effect at these endpoints was recorded by the following laboratory and instrumental parameters: a qualitative evaluation of the urine cellular an according to the Nechiporenko test, maximum flow rate (Qmax) and form of the uroflowgram, prostate volume, post-void residual based on transabdominal ultrasound investigation, and the plasma level of total PSA.
Description of the drug studied. Gardaprost® is a natural herbal complex. A standard capsule (400 mg) contains green tea extract (Camellia sinensis) – 120 mg (including epigalocatechin-3-gallate – 75 mg), curcumin > 25 mg, genistein (soybean-derived) > 65 mg. Excipients: gelatine. One pack contains 30 capsules.
Statistical analysis. The analysis of the screening and control parameters in both groups for the type of distribution was carried out using the Kolmogorov-Smirnov test. Statistical analysis of the results was performed using IBM SPSS Statistica 23 software (StatSoft Inc., IBM SPSS Corp., Tulsa, OK, USA). The mean values were presented as the arithmetical mean, and the mean deviations were presented as the mean square deviation. Intergroup differences in normally distributed variables were evaluated with the t-test for paired samplings and ANOVA. For variables with non-normal distribution, a Friedman two-factor ANOVA on ranks was used for associated samplings. The level of significance was > 95%.
Results
At the time of inclusion in the study, the mean age of patients in the main group was 63.5 ± 2.9 years old, which was comparable with the control group 64.4 ± 5.2 years old (p = 0.403). Besides, there were no differences in the prostate volume, post-void residual, maximum flow rate (Qmax), total IPSS and IIEF-5 scores, the number of daytime and nocturnal urination acts, the volume of consumed liquid, and the PSA level in the main and control groups, respectively (p > 0.05) (Table 1).
Table 1. Comparative demographics of groups after randomization
|
Parameter |
Follow-up groups |
p |
|
|
main Tamsulosin + Gardaprost® |
control Tamsulosin |
||
|
Age, years |
63.5 ± 2.9 |
64.4 ± 5.2 |
0.403 |
|
Prostate volume, cm3 |
65.3 ± 12.05 |
72.5 ± 24.1 |
0.208 |
|
Residual urine volume, ml |
64.4 ± 10.99 |
79.1 ± 27.2 |
0.130 |
|
Maximum flow rate, ml/s |
9.7 ± 1.3 |
10.5 ± 2.1 |
0.106 |
|
Total I-PSS score, points |
23.8 ± 2.5 |
23.7 ± 3.0 |
0.867 |
|
Total IIEF-5 score, points |
15.6 ± 5.0 |
18.3 ± 3.2 |
0.170 |
|
Daily urination, n |
7.6 ± 1.4 |
7.8 ± 1.9 |
0.703 |
|
Nocturnal urination / nocturia, n |
1.964 ± 0.96 |
1.963 ± 0.8 |
0.996 |
|
Fluid in-take, ml |
1675 ± 275.4 |
1428.6 ± 285.3 |
0.125 |
|
PSA, ng/ml |
4.1 ± 1.6 |
6.2 ± 2.5 |
0.100 |
Notes: PSA — prostate-specific antigen; IPSS — International Prostate Symptom Score;
IIEF-5 — International Index of Erectile Function
In both groups, treatment was associated with a significant decrease in LUTS expression six- and twelve-months follow-up (IPSS). In the control group, the respective parameters scored 18.6 ± 2.8 and 19.7 ± 7.2, which was 5.1 and 4.0 score points lower than the baseline value (p < 0.01) (Fig. 1). Therapy with tamsulosin in combination with Gardaprost® was associated with a significantly more expressed positive dynamic in this group of observation. There was a reduction in the IPSS total score by 8.2 and 16.1 points to become 15.6 ± 3.7 and 7.9 ± 2.1 in six and 12 months, respectively (p < 0.001) (Fig. 1). This provided significant differences between the studied groups in the endpoints (p < 0.01) (Fig. 1).
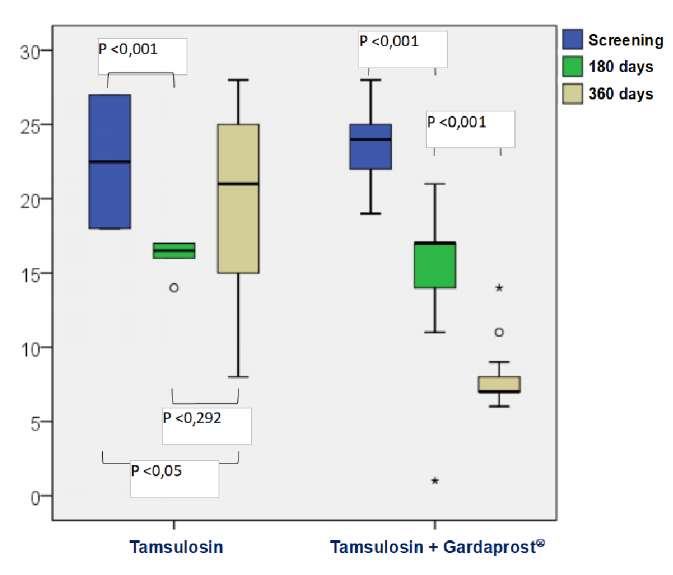
Figure 1. Dynamics of lower urinary tract symptoms severity during treatment
at the inclusion stage, after 6 and 12 months of therapy according to the total IPSS score
Monotherapy with tamsulosin did not significantly influence the number of daytime urination and nocturia acts during the observation period. On the screening endpoints on Days 180 and 360, they were 7.8 ± 1.9, 7.96 ± 2,1 and 8.6 ± 2.1, and 2.0 ± 0.8, 1.7 ± 0.9, and 1.9 ± 1, respectively (p > 0.05) (Table 2). On the contrary, in the group of combined therapy, there was a positive dynamic in both parameters. The best tendency was observed in the resolution of the nocturia. In the analog time intervals, the rate of nocturnal urinations was 1.964 ± 0.96, 1.1 ± 0.8, and 0.3 ± 0.5, which had statistically significant differences within the main group of observation and in relation to the control group (p < 0.01) (Table 2). Similar but weaker expressed tendencies were observed in relation to the rate of daytime urinations in the main group (p < 0.05) (Table 2). It should be mentioned that there were no differences in fluid intake and daily diuresis in the follow-up groups during the study (p > 0.05) (Table 3).
Table 2. Dynamics of the frequency of daily and night urinations in the follow-up groups
at the inclusion stage, after 6 and 12 months of therapy
|
Follow-up periods |
p** |
|||||
|
screening |
180 days |
360 days |
screening vs 180 days |
screening vs 360 days |
180 days vs 360 day |
|
|
Daily urinations, n |
||||||
|
Tamsulosin |
7.8 ± 1.9 |
7.96 ± 2.1 |
8.6 ± 2.1 |
0.655 |
0,068 |
0,147 |
|
Tamsulosin + Gardaprost® |
7.6 ± 1.4 |
5.9 ± 1.0 |
5.3 ± 0.9 |
< 0.001 |
< 0,001 |
0,004 |
|
p* |
0.703 |
< 0.001 |
< 0.001 |
|||
|
Night urinations, n |
||||||
|
Tamsulosin |
1.96 ± 0.8 |
1.7 ± 0.9 |
1.9 ± 1.0 |
0.11 |
0.852 |
0.379 |
|
Tamsulosin + Gardaprost® |
1.96 ± 0.96 |
1.1 ± 0.8 |
0.3 ± 0.5 |
< 0.001 |
< 0.001 |
0.001 |
|
p* |
0.996 |
0.007 |
< 0.001 |
|||
Note: p* – differences between groups, p** – in-group differences
Table 3. Dynamics of the fluid intake and daily urine output in the follow-up groups
at the inclusion stage, after 6 and 12 months of therapy
|
Follow-up periods |
p** |
|||||
|
screening |
180 days |
360 days |
screening vs 180 days |
screening vs 360 days |
180 days vs 360 day |
|
|
Fluid intake, ml |
||||||
|
Tamsulosin |
1428.6 ± 285.3 |
1442.9 ± 426.1 |
1509.05 ± 308.6 |
0.845 |
0.197 |
0.438 |
|
Tamsulosin + Gardaprost® |
1675 ± 275.4 |
1750 ± 288.7 |
1662.5 ± 193.1 |
0.744 |
0.942 |
0.213 |
|
p* |
0.125 |
0.184 |
0.352 |
|||
|
Daily urine output, ml |
||||||
|
Tamsulosin |
1290.4 ± 451.6 |
1290.4 ± 489.92 |
1353.1 ± 469.3 |
1 |
0.318 |
0.321 |
|
Tamsulosin + Gardaprost® |
1390.4 ± 367.1 |
1263.8 ± 579.64 |
1240.8 ± 562.5 |
0.145 |
0.111 |
0.658 |
|
p* |
0.43 |
0.867 |
0.465 |
|||
Note: p* – differences between groups, p** – in-group differences
Initially, patients from the main group had more expressed erectile dysfunction, the total IIEF-5 score was 15.6 ± 5 versus 18.3 ± 3.2 in the control group (p = 0.017) (Table 4). In the main group, there was a tendency for an enhancement of erectile function, which was shown by an increase in the IIEF-5 score to 16.7 ± 4.3 and 20 ± 4.4 in the respective control points (p < 0.001). In the control group, there were no dynamic changes in this parameter 18.3 ± 3.2, 18.2 ± 4.4, and 17 ± 3.5 points (p > 0.05, Table 4). Thus, by the end of the experiment, there were no differences in the IIRF-5 score in the groups (p = 0.15) (Table 4) and the rate of anejaculation, which was 19.2 and 20% in the control and main group, respectively (p > 0.05).
Table 4. International index of erectile function in the follow-up groups
at the inclusion stage, after 6 and 12 months of therapy
|
Follow-up period |
p** |
||||||
|
screening |
180 days |
screening |
180 days |
screening |
180 days |
||
|
Tamsulosin |
18.3 ± 3.2 |
18.2 ± 4.4 |
17 ± 3.5 |
0.862 |
0.081 |
0.084 |
|
|
Tamsulosin + Gardaprost® |
15.6 ± 5.0 |
16.7 ± 4.3 |
20 ± 4.4 |
0.282 |
< 0.001 |
0.002 |
|
|
p* |
0.017 |
0.207 |
0.15 |
||||
Note: p* – differences between groups, p** – in-group differences
The IPSS score showed that the positive clinical dynamics in the control group did not correlate with the uroflowmetric parameters. Thus, the maximum rate of urine flow did not change in the control group during the follow-up period (p > 0.05) (Fig. 2). In the main group, in six months, the maximal rate of urination increased by 28.6%, and in 12 months, by 83.7%, which was 9.8 ± 1.3 ml/s, 12.6 ± 1.4 ml/s, and 18.0 ± 7.3 ml/s in the control points, respectively (p < 0.05) (Fig. 2). Besides, there were significant differences between the main and control groups after the beginning of the therapy according to ANOVA results. Therefore, during the screening visit, the significance criterion did not show any differences between the groups p = 0.106, at six and twelve months, it was p = 0.00112 and p = 0.00001 (Fig. 2).
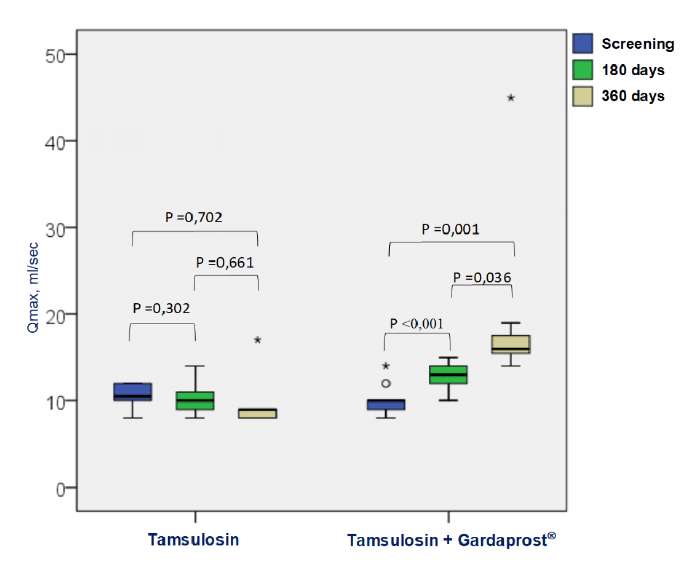
Figure 2. Dynamics of the maximum urination flow rate (Qmax) in the main and control groups
In both groups, there was a positive dynamic by the post-void residual urine, which was observed at the last endpoint and characterized by a significant decrease in the post-void residual urine in comparison with the screening visit. After six months of tamsulosin therapy, the rate of mean volume of post-void residual urine decreased by 24% from 79.1 ± 27.2 to 60.2 ± 32.5 ml (p = 0.005) (Fig. 3), and by the end of the study, there was an insignificant increase in this parameter, which was 65.9 ± 33.2 ml (p = 0.105) (Fig. 3), which was 17% lower than during the screening visit and these differences remain significant (p < 0.043) (Fig. 3). In the main group, the volume of post-void residual urination decreased by 36% and 63%, respectively (64.4 ± 10.99 vs 41.1 ± 21.9 vs 23.6 ± 13.6 ml; p < 0.001) during the same period (Fig. 3).
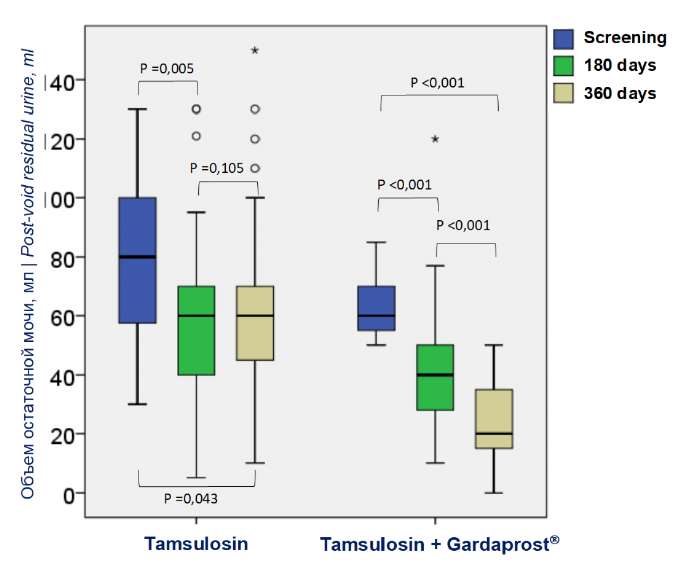
Figure 3. Dynamics of post-void residual urine (ml)
while taking tamsulosin and tamsulosin + Gardaprost®
There were no differences in prostate volume between the groups (p = 0.208) (Table 1). Gardaprost® therapy significantly reduced prostate volume. In the main group, it decreased significantly by 18.8% (65.7 ± 11.9 vs 53.3 ± 8.0 ml; p < 0.001) in 12 months after the screening visit (Fig. 4). In the control group, prostate volume did not change significantly during the period of observation and was 72.15 ± 24.1 vs 74.2 ± 24.4 (p = 0.1) (Fig. 4).
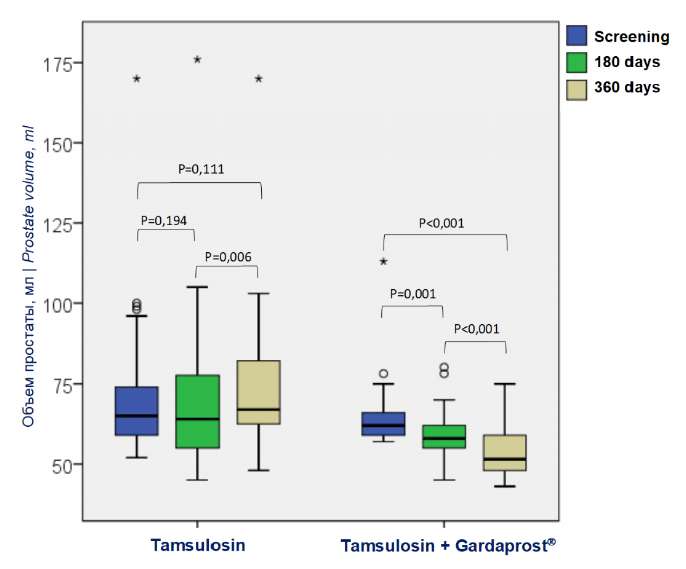
Figure 4. Dynamics of prostate volume (ml)
while taking tamsulosin and tamsulosin + Gardaprost ® for 12 months
Discussion
The pathogenesis of BPH and the associated LUTS remains unclear. It is believed that LUTS pathogenesis includes mechanical obstruction by hypertrophic prostate, dynamic obstruction caused by bladder neck and prostatic urethral stenosis, and inflammatory changes in the prostate [1][3]. The results of multicenter studies showed that the latter acted as predictors of the disease progression and the risk of surgical treatment [2][4][6]. The application of alfa-1-adrenoblockers is the most popular option for BPH therapy, which is associated with a fast and stable 1/3 decrease in the expression of LUTS [1][5]. However, the application of alfa-1-adrenoblockers does not reduce the risk of surgical interventions. Currently, it is known that combined pharmacotherapy with drugs of various mechanisms of action is the optimal option. The most well-studied combination in this aspect is alfa-1-adrenoblockers and 5-alpha reductase inhibitors [2]. Another important moment is that along with the summation of positive effects, a similar tendency was observed also in side effects like cardiovascular, erectile, and ejaculatory disorders, gynecomastia, dizziness, head, and chest pain [6]. Thus, the search for effective and safe drugs to treat LUTS associated with BPH is an acute problem in modern urology.
In the present study, a one-year combined therapy for BPH with tamsulosin and Gardaprost® showed more advantages in comparison with tamsulosin monotherapy. The authors believe that additional bonus from the application of Gardaprost® was associated with the anti-proliferative, anti-inflammatory, and anti-cytokine effects of its components.
A molecule of curcumin, included in the formulation, suppresses cells proliferation, and enhances apoptosis [10]. These effects can be explained by the suppressing effect on hypoxia-induced factor 1 alfa (HIF-1α), which was proposed as a key molecule of BPH pathogenesis. The expression of HIF-1α on cellular membranes is mediated by the secretion of anti-inflammatory cytokines [10]. Other authors showed that curcumin was a potent blocker of tumor necrosis factor (TNF) and lipoxygenase-1 (LOX-1). The anti-proliferative effect can also be associated with a decrease in the expression of transforming growth factor (TGF) and insulin-like growth factor (IGF) [11].
Another component in the formulation is genistein, which is a typical phytoestrogen compound chemically homologous to estradiol. Genistein initiates apoptosis and cytotoxic processes, and in a dose-dependent manner decreases the growth of BPH and prostate adenocarcinoma [12]. In vitro studies showed that genistein suppressed the proliferation of androgen-dependent and hormone-resistant cell culture in the human prostate [13].
The third ingredient in the formulation is epigallocatechin-3-gallate. The studies showed that epigallocatechin suppressed cell proliferation and induced apoptosis in cancerous cells by affecting angiogenesis processes. It inhibits the activation of some types of receptor tyrosine kinase (EGF) [14]. Another pathway of realization of anti-proliferative properties of epigallocatechin is inhibition of metastatic tumors and initiation of caspase-dependent and caspase-independent apoptosis because of an increase in lysosomal membrane permeability and autophagy [15]. Epigallocatechin also contributes to a decrease in the production of reactive oxygen and nitrogen intermediates suppressing the secretion of anti-inflammatory cytokines and showing anti-inflammatory effects [16]. In addition, there are data on organ-specific suppression of cellular proliferation in patients with BPH, which was associated with disturbances in the organization of the cytoskeleton and interactions in the extracellular matrix [17].
The authors suggest that the obtained positive results in the main group are associated with anti-proliferative, proapoptotic, and anti-inflammatory effects of the components of Gardaprost®, which was shown by a significant decrease in prostate volume a year after the application of the drug. This provided better clinical and urodynamic parameters in this group. The authors did not register any cross-side effects that are typical for 5-alpha reductase inhibitors either because Gardaprost® does not affect testosterone hydroxylation or because of a small sampling.
Conclusion
The authors obtained promising long-term results of the application of Gardaprost® in combined therapy for moderate and expressed LUTS associated with medium and large volume of BPH. Placebo-controlled studies with larger samples should be conducted to obtain a high-level recommendation base and assess the safety profile of this therapy.
References
1. Ergakov D.V., Martov A.G. Lower urinary tract symptoms due to prostatic hyperplasia in 2017: updates from the 32nd Congress of the European Association of Urology. Urologiia. 2017;3-S3:36-44. (In Russ.). DOI: 10.18565/urol.2017.3-supplement.36-44.
2. Vinogradov I.V. Evaluation of the efficacy and tolerability of combination therapy for lower urinary tract symptoms in patients with chronic prostatitis and benign prostatic hyperplasia. Experimental and Clinical Urology. 2021;14(1):37-43. (In Russ.). DOI: 10.29188/2222-8543-2021-14-1-37-42.
3. Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2019;22(1):12-19. DOI: 10.1080/13685538.2018.1434772.
4. Startsev V.Yu., Dudarev V.A., Sevryukov F.A., Zabrodina N.B. Economic aspects of the treatment of patients with urination disorders caused by benign prostatic hyperplasia. Urologiia. 2019;(6):115-119. (In Russ.). DOI: 10.18565/urology.2019.6.115-119.
5. Pushkar D.Yu., Rasner P.I. Lower urinary tract symptoms and benign prostatic hyperplasia. Urologiia. 2018;(S1):30-45. (In Russ.). eLIBRARY ID: 36320172.
6. Oelke M, Becher K, Castro-Diaz D, Chartier-Kastler E, Kirby M, Wagg A, Wehling M. Appropriateness of oral drugs for long-term treatment of lower urinary tract symptoms in older persons: results of a systematic literature review and international consensus validation process (LUTS-FORTA 2014). Age Ageing. 2015;44(5):745-55. DOI: 10.1093/ageing/afv077.
7. Gilyazov A.Kh., Asubaev A.G., Akhmetova K.Kh., Khutiev C.Ya., Tereshkin C.V., Меуеr Т.Е., ProKofiev A.Yu., Bodesova C.B. Phytotherapy in the treatment of benign prostatic hyperplasia (BPH) with the medication Himplaziya. Meditsina (Almaty). 2014;2(140):54-56. (In Russ.). eLIBRARY ID: 37034705.
8. Fornara P, Madersbacher S, Vahlensieck W, Bracher F, Romics I, Kil P. Phytotherapy Adds to the Therapeutic Armamentarium for the Treatment of Mild-To-Moderate Lower Urinary Tract Symptoms in Men. Urol Int. 2020;104(5-6):333-342. DOI: 10.1159/000504611.
9. Bhatt NR, Davis NF, Witjes WP, Bjartell A, Caris C, Patel A, de la Taille A, Speakman M, Martínez-Piñeiro L, Tubaro A. Contemporary use of phytotherapy in patients with lower urinary tract symptoms due to benign prostatic hyperplasia: results from the EVOLUTION European registry. World J Urol. 2021;39(7):2661-2667. DOI: 10.1007/s00345-020-03480-w.
10. Kim HJ, Park JW, Cho YS, Cho CH, Kim JS, Shin HW, Chung DH, Kim SJ, Chun YS. Pathogenic role of HIF-1α in prostate hyperplasia in the presence of chronic inflammation. Biochim Biophys Acta. 2013;1832(1):183-94. DOI: 10.1016/j.bbadis.2012.09.002.
11. Kim SK, Seok H, Park HJ, Jeon HS, Kang SW, Lee BC, Yi J, Song SY, Lee SH, Kim YO, Chung JH. Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement Altern Med. 2015;15:380. DOI: 10.1186/s12906-015-0825-y.
12. Klein CB, King AA. Genistein genotoxicity: critical considerations of in vitro exposure dose. Toxicol Appl Pharmacol. 2007;224(1):1-11. DOI: 10.1016/j.taap.2007.06.022.
13. Geller J, Sionit L, Partido C, Li L, Tan X, Youngkin T, Nachtsheim D, Hoffman RM. Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture. Prostate. 1998;34(2):75-9. DOI: 10.1002/(sici)1097-0045(19980201)34:2<75::aid-pros1>3.0.co;2-i.
14. Shimizu M, Sakai H, Shirakami Y, Yasuda Y, Kubota M, Terakura D, Baba A, Ohno T, Hara Y, Tanaka T, Moriwaki H. Preventive effects of (-)-epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db Mice. Cancer Prev Res (Phila). 2011;4(3):396-403. DOI: 10.1158/1940-6207.CAPR-10-0331.
15. Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, Nishizaki T. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002;956(2):319-22. DOI: 10.1016/s0006-8993(02)03564-3.
16. Chu C, Deng J, Man Y, Qu Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. Biomed Res Int. 2017;2017:5615647. DOI: 10.1155/2017/5615647.
17. Tepedelen BE, Soya E, Korkmaz M. Epigallocatechin-3-gallate reduces the proliferation of benign prostatic hyperplasia cells via regulation of focal adhesions. Life Sci. 2017;191:74-81. DOI: 10.1016/j.lfs.2017.10.016.
18.
About the Authors
S. V. ShkodkinRussian Federation
Sergey V. Shkodkin — M.D., Dr.Sc.(Med), Assoc.Prof.(Docent); Prof., Dept. of Hospital Surgery; Urologist
Belgorod
M. V. Pokrovskiy
Russian Federation
Mikhail V. Pokrovsky — M.D., Dr.Sc.(Med), Full Prof.; Head, Dept. of Pharmacology, Head, Centre for Preclinical and Clinical Research
Belgorod
S. S. Krasnyak
Russian Federation
Stepan S. Krasnyak — M.D., Cand.Sc.(Med); Researcher, Dept. of Andrology and Human Reproduction
Moscow
A. V. Polishchuk
Russian Federation
Alexey V. Polichuk — M.D.; Assist., Dept. of Hospital Surgery; Urologist
Belgorod
S. V. Chirkov
Russian Federation
Sergey V. Chirkov — M.D.; Postgraduate Student, Dept. of Hospital Surgery; Urologist, Outpatient Clinic No. 3
Belgorod
O. V. Churikova
Russian Federation
Olga V. Churikova — M.D.; Applicant, Dept. of Hospital Surgery; Urologist
Belgorod
N. A. Kravtsova
Russian Federation
Natalya A. Kpavtsova — M.D.; Urologist
Belgorod
Review
For citations:
Shkodkin S.V., Pokrovskiy M.V., Krasnyak S.S., Polishchuk A.V., Chirkov S.V., Churikova O.V., Kravtsova N.A. Combination therapy for benign prostate hyperplasia-related urinary symptoms. Urology Herald. 2022;10(1):84-95. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-84-95













































