Scroll to:
PSA density as a predictor of long-term infectious complications during transurethral resection of the prostate: determining an optimal cut-off value
https://doi.org/10.21886/2308-6424-2024-12-1-19-26
Abstract
Introduction. Infectious complications (ICs) after transurethral resection of the prostate (TURP) are significant and potentially life-threatening conditions with an incidence 0.5% – 20.0%. Most publications provide data regarding early infectious complications. At the same time, there are currently no studies aimed at a comprehensive assessment of long-term infectious complications after TURP. The problem of prevention and treatment of ICs is also accompanied by insufficient understanding of the role of undiagnosed inflammation in prostate tissues and the lack of representative laboratory markers.
Objective. To assess the prostate-specific antigen density (PSAd) as a predictor of long-term infectious complications after transurethral resection of the prostate and to determine the optimal cut-off value.
Materials & methods. This single-center study included 162 patients who underwent mono- and bipolar TURP between 2016 – 2023. Inclusion criteria for the study: prostate volume 30–80 cm3, no history of urinary tract infections (UTIs) at the time of hospitalization and antibiotic treatment at least one month before surgery, possible presence of latent UTIs before surgery, no prostate cancer. Exclusion criteria were failure to meet inclusion criteria. Infectious complications assessed included upper and lower UTIs, as well as epididymitis, orchitis and prostatitis, confirmed by clinical and laboratory data. Prostate-specific antigen (PSA) assessment was performed < 2 days before surgery.
Results. The median PSAd value was 0.04 [0.03; 0.08] ng/ml2, the variable was significantly different in non-infection and infection groups (0.04 and 0.08 ng/ml2, respectively, p = 0.009). The area under the curve (AUC) was 0.67 (95% CI [0.546 – 0.791]). The optimal cut-off value of the PSAd in prediction of long-term ICs was > 0.07 ng/ml2, sensitivity / specificity: 58.3% and 76.1%, respectively. The analysis showed more than 4 times higher odds of developing an infectious complication in PSAd > 0.07 ng/ml2 patients: OR 4.3 (95% CI [1.7 – 10.5], p = 0.001).
Conclusion. This study demonstrates data that defines a new clinical non-oncological significance of PSAd as a predictor of the development of long-term infectious complications after TURP.
Keywords
For citations:
Ivanov S.N., Kogan M.I., Naboka Yu.L., Medvedev V.L., Palaguta G.A. PSA density as a predictor of long-term infectious complications during transurethral resection of the prostate: determining an optimal cut-off value. Urology Herald. 2024;12(1):19-26. https://doi.org/10.21886/2308-6424-2024-12-1-19-26
Introduction
Transurethral resection of the prostate (TURP) is one of the most common surgical procedures in urology and is considered the gold standard for surgical treatment of benign prostatic hyperplasia (BPH) [1][2]. Infectious complications (ICs) are the most dangerous limitations of endourological prostate surgery. ICs are potentially life-threatening difficult to control conditions and one of the leading causes of sepsis. The incidence of infectious complications during transurethral prostate surgery ranges from 0,5 to 20,0% [2-5]. Most studies provide data regarding immediate and short-term infectious complications. At the same time, there are currently no publications providing comprehensive assessment of long-term infectious complications after TURP. The problem of prevention and treatment of ICs is also complicated by insufficient understanding of the role of undetected inflammation in prostate tissues and the lack of representative laboratory markers.
Objective. To assess the prostate-specific antigen density (PSAd) as a predictor of long-term infectious complications after transurethral resection of the prostate.
Materials and methods
This single-center study included 162 patients who underwent mono- and bipolar TURP between 2016 and 2023 with monopolar 24 Fr and bipolar 24 Fr resectoscopes Karl Storz («Karl Storz SE GmbH & Co. KG», Tuttlingen, Germany). Inclusion criteria for the study: prostate volume 30 – 80 cm3, no history of symptomatic urinary tract infections (UTIs) at the time of hospitalization and treatment with antibacterial drugs at least one month before surgery, possible presence of latent UTIs, absence of prostate cancer. The exclusion criterion was failure to meet the inclusion criteria.
Preoperative examination of patients was carried out in accordance with clinical guidelines. Prostate volume was assessed using transrectal ultrasound (ml), device — RS80A (Samsung, South Korea), probe — CA2-9A (2 – 9 MHz). Total blood PSA was assessed no more than two days before surgery. PSA density (ng/ml2) was determined using the formula = PSA (ng/ml) / prostate volume (ml). All patients with a total blood PSA level > 4 ng/ml, as well as having digital rectal examination or magnetic resonance imaging (magnetic resonance imaging scanner GE Discovery MR750w 3.0T, General Electric, USA) underwent prostate biopsy at least three months before proposed transurethral prostate surgery in in the presence of indications: a total blood PSA value > 4 ng/ml, digital rectal examination, and magnetic resonance imaging data suspicious for prostate cancer. The number of daytime and nighttime voids was assessed by completing a voiding diary for 7 days. The development of long-term ICs was defined as clinical and laboratory signs of upper and lower urinary tract infections or in presence of confirmed episodes of epididymitis, orchitis and prostatitis.
Statistical analysis. Statistical analysis was carried out in the statistical data processing environment IBMÒ SPSS Statistics ver. 23.0 («SPSS: An IBM Company», IBM SPSS Corp., Armonk, NY, USA). The Kolomgorov-Smirnov-Lilliefors test was used to test the distribution normality and the equality of variance was also assessed. Descriptive statistics for quantitative variables are presented for normal distribution as the mean (M) and standard deviation (±SD) and for non-normal distribution as the median (Me) and interquartile range [Q1; Q3]. To compare quantitative variables with normal and non-normal distribution, Student t-test + Levene test and Mann-Whitney U test were used respectively. Frequencies were compared using Pearson’s chi-square with Yates's correction. For correlation analysis, the Spearman rank correlation coefficient was used. Logistic regression analysis was used to assess the predictive power of the variable. Nagelkerke R2 was used to calculate the proportion of variance in clinical outcomes that could be explained by the predictors. Receiver operating characteristics (ROC) curves and the corresponding area under the ROC–AUC curve were used to assess the quality of the model. A sample size of 162 patients is enough to achieve an effect size of 80% for logistic regression.
Results
1) Analysis of demographic details and baseline perioperative data.
Baseline characteristics and a comparative analysis of groups with and without ICs are presented in Table 1. The median follow-up was 4 [ 1.2; 5.4] years. ICs were developed in 24 (14.8%) patients during follow-up: urethritis developed in three cases, epididymo-orchitis in nine cases, acute prostatitis in one case, and cystitis in 11 cases. Age, BMI, prostate volume, daytime and nighttime urination frequency before surgery and the incidence of early postoperative ICs during hospitalization were comparable in groups. The groups had statistically significant differences in baseline total blood PSA and PSA density.
Table 1. Demographic details and baseline
|
Variables |
Total (n = 162) |
Non-infection (n = 138) |
Infection (n = 24) |
р |
|
|
Age, M ± SD years |
67.5 ± 7.1 |
67.5 ± 6.9 |
67.1 ± 7.9 |
0.775 |
|
|
BMI, M ± SD kg/m2 |
28.5 ± 4.2 |
28,5 ± 4.2 |
28.4 ± 4.2 |
0,907 |
|
|
Diabetes, n (%) |
28 (17.3) |
26 (18.8) |
2 (8.3) |
0.335 |
|
|
Suprapubic drainage, n (%) |
39 (24.1) |
31 (22.5) |
8 (33,3) |
0.373 |
|
|
Prostate volume, M ± SD ml |
71.0 ± 23.5 |
71.3 ± 23,6 |
69.2 ± 23.1 |
0.689 |
|
|
Total PSA, Me [Q1; Q3] ng/ml |
3.2 [ 1.8; 6.4] |
3.1 [ 1.8; 5.2] |
4,4 [ 2.7; 10.8] |
0.043* |
|
|
PSAd, Me [Q1; Q3] ng/ml2 |
0.04 [ 0.03; 0.08] |
0.04 [ 0.03; 0.07] |
0.08 [ 0.04; 0.11] |
0.009* |
|
|
Day urination, M ± SD |
6.4 ± 1.3 |
6.3 ± 1.3 |
6.5 ± 1.2 |
0.531 |
|
|
Night urination, M ± SD |
4.6 ± 2.3 |
4.5 ± 2.3 |
5.0 ± 2.3 |
0.335 |
|
|
Early IC, n (%) |
19 (11.7) |
16 (11.6) |
3 (12.5) |
1.000 |
|
|
Long-term IC, n (%) |
24 (14.8) |
0 (0.0) |
24 (100.0) |
– |
|
|
Notes. 1) * differences are significant at p < 0.05; Student's t test + Levene's test and Mann-Whitney U test were used to compare quantitative variables for data with and without normal distribution, respectively; frequencies were compared using Pearson's chi-square + Yates's correction 2) PSA — prostate-specific antigen; PSAd — PSA density; BMI — body mass index; IC — infectious complications; M ± SD — mean ± standard deviation; Me [Q1; Q3] — median & interquartile range |
|||||
Table 2 presents the correlations between baseline total PSA and PSA density values and the incidence of long-term ICs.
Table 2. PSA and PSA density correlations with the incidence of infectious complications
|
Variables |
Long-term IC |
|
|
r |
p |
|
|
Total PSA, ng/ml |
159 |
0.043 |
|
PSA density, ng/ml2 |
207 |
0.008 |
|
Notes. 1) r — Spearman correlation coefficient, statistically significant at p < 0.05 2) IC — infectious complications; PSA — prostate-specific antigen |
||
Figure 1 shows the distribution of PSA density by occurrence frequency (%). About 85.2% of values did not reach 0.10 ng/ml2, 10.5% of values were in the range of 0.10 – 0.20 ng/ml2, 1.9% were in the range of 0.20 – 0.25 ng/ml2, 2.5% – represented by values of 0.35 – 0.50 ng/ml2.
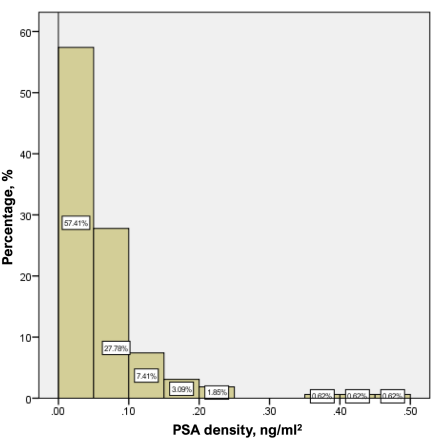
Figure 1. Frequency distribution of PSA density (PSAd) values
2) ROC analysis of the prognostic value of PSA density as a predictor of long-term infectious complications.
The median PSA density was 0.04 [ 0.03; 0.08] ng/ml2. The area under the curve (AUC) was 0.67, standard deviation 0.062 (95% CI [ 0.546 – 0.791]) (Fig. 2).
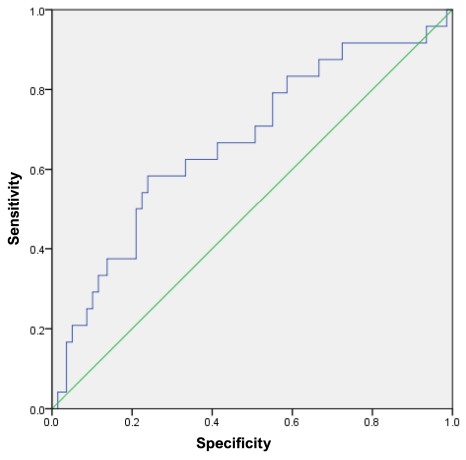
Figure 2. ROC curve of PSA density for the outcome: long-term infectious complications. ROC — Receiver Operating Characteristic
3) Optimal cut-off value of the PSA density for predicting distant infectious complications.
From a sequential series of PSAd values [min: 0.0049 ng/ml2; max: 5.058 ng/ml2] the range with the optimal ratio of sensitivity (54.2% – 58.3%) and specificity (73.9% – 77.5%) was determined: from 0.06 ng/ml2 to 0.08 ng /ml2 (in Fig. 3 the range is limited by the red dotted lines). The cut-off value PSAd is located at the intersection of the green dashed lines.
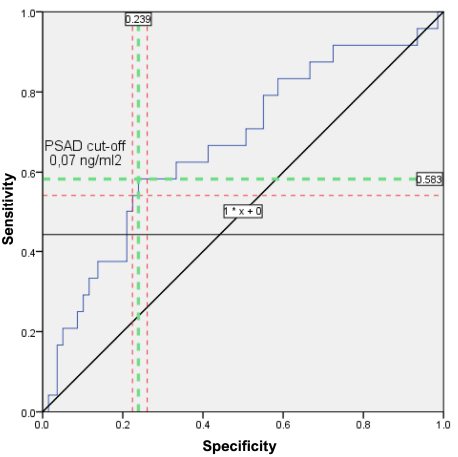
Figure 3. PSAd cut-off value on the ROC curve for the outcome: long-term infectious complications. (PSAd — prostate-specific antigen density; red dotted lines limit the optimal range of sensitivity and specificity; PSAd cut-off value is at the intersection of the green dashed lines. ROC — Receiver Operating Characteristic)
Table 3 presents the sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, coefficients of determination (Nagelkerke R2), and predictor significance (p) at different cut-off levels. The optimal ratio of sensitivity / specificity, positive / negative predictive value, as well as the highest percentage of explained variation in outcome (long-term ICs) was determined for a PSAd cut-off value > 0.07 ng/ml2.
Table 3. Predictive accuracy of different PSA density cut-off values for assessing the risk of long-term infectious complications
|
PSAd cut-off value, ng/ml2 |
Sensitivity, (%) |
Specificity, (%) |
PPV, (%) |
NPV, (%) |
LR+, (%) |
LR–, (%) |
R2 |
p |
|
> 0.06 |
58.3 |
73.9 |
90.6 |
25.0 |
2.23 |
56.5 |
0.071 |
0.010* |
|
> 0.07 |
58.3 |
76.1 |
91.2 |
29.2 |
2.43 |
58.2 |
0.107 |
0.002* |
|
> 0.08 |
54.2 |
77.5 |
90.9 |
29.3 |
2.40 |
59.0 |
0.086 |
0.004* |
|
Note. PSAd — prostate-specific antigen density; PPV / NPV — positive / negative predictive value; LR+ / LR- — positive/negative likelihood ratio; * statistically significant at p < 0.05 |
||||||||
4) Goodness-of-fit analysis for univariate models predicting long-term infectious complication from PSAd values.
ROC curves of predicted and observed IC outcomes were constructed for three cut-off values including the optimal PSAd > 0.07 ng/ml2 to perform a goodness-of-fit analysis of the univariate model. Models’ characteristics are presented in Table 4. The analysis confirmed the highest predictive value of the optimal cut-off value PSAd > 0.07 ng/ml2, AUC = 0.67; 95% CI [ 0.55 – 0.79].
Table 4. Goodness-of-fit test for univariate models predicting long-term infectious complications from PSAd values
|
PSAd cut-off value |
AUC |
CI lower limit |
CI upper limit |
p |
|
> 0.06 |
0.639 |
0.516 |
0.763 |
0.029* |
|
> 0.07 |
0.668 |
0.545 |
0.792 |
0.009* |
|
> 0.08 |
0.645 |
0.517 |
0.773 |
0.024* |
|
Note. PSAd — prostate-specific antigen density; AUC — area under the curve; CI — confidence interval; *statistically significant at p < 0.05 |
||||
5) Assessment of the relationship between long-term ICs and PSA density.
To assess the relationship the odds ratio (OR) was calculated for a cut-off value of PSAd > 0.07 ng/ml2. The analysis showed a more than 4 times increase in the odds of developing an infectious complication with an increase in PSAd > 0.07 ng/ml2: OR = 4.3, 95% CI [ 1.7 – 10.5], p = 0.001.
Discussion
Most studies do not discuss PSA level as a risk factor for the development of infectious complications after TURP [6-11]. According to recent studies about 10.0 – 12.0% of the results of PSA assessment as part of cancer screening are false positive [12]. Discussion of the high rate of false-positive results in total PSA screening tests primarily focuses on overdiagnosis, over-prescription of biopsies, and biopsy-associated complications. From our point of view, it is important to focus attention on the causes of non-cancer-related PSA variability and false-positive results of PSA screening. PSA is nonspecific for assessing the oncological process and is reactive in inflammatory processes in the prostate. It is noted that febrile UTIs in men may be accompanied by an increase in serum PSA values [13]. In a 2023 meta-analysis based on pooled data from three studies (290 patients) our scientific group confirmed that the average preoperative PSA values is significantly higher in patients with postoperative bacteriuria [14]. The present investigation continued the research trend on the PSA role in the issue of infectious status of patients undergoing TURP and confirmed the association between PSA values and long-term ICs.
It is known that PSA values associated with the prostate volume [15]. In current study, it was decided to assess PSAd to increase PSA representativeness as a marker of prostate inflammatory status. The results of the analysis showed a more significant relationship between the PSAd and long-term ICs compared with the total blood PSA. These findings allow us to propose the use of this biomarker as an important predictor of ICs. Our results demonstrate the potential role of PSAd as a marker of undetected inflammatory processes in the prostate. To our knowledge, this is the first study to demonstrate the non-cancer prognostic PSAd values in determining the risk of long-term infectious complications after TURP.
Study limitations included the small sample size, single-centre, and retrospective study design.
Conclusion
This study demonstrates data that defines a new clinical non-oncological significance of PSAd as a predictor of the development of long-term infectious complications after transurethral resection of the prostate. The results of this study suggest further research into the structure of significant predictors of infectious complications in the early and long-term postoperative periods and the development of multivariate prognostic models.
Key points:
- This study suggests the significance of undetected inflammatory processes in prostate tissue as a factor in the development of long-term infectious complications.
- Current study supports the need for a more in-depth assessment of the prostate infection and inflammatory status to improve the safety of endosurgical interventions.
- The theoretical significance of the research is to confirm the correlation between PSA values and the development of infectious complications.
- The proposal to calculate PSA density as a volume-adjusted index achieved greater prognostic value for predicting infectious complications with prostate interventions
References
1. Jo JK, Shinn SH, Kim KS, Moon HS. Changes in Prevalence and Treatment Pattern of Benign Prostatic Hyperplasia in Korea. Int Neurourol J. 2021;25(4):347-354. DOI: 10.5213/inj.2040412.206
2. Lin YH, Hou CP, Chen TH, Juang HH, Chang PL, Yang PS, Chen CL, Tsui KH. Transurethral resection of the prostate provides more favorable clinical outcomes compared with conservative medical treatment in patients with urinary retention caused by benign prostatic obstruction. BMC Geriatr. 2018;18(1):15. DOI: 10.1186/s12877-018-0709-3
3. Vivien A, Lazard T, Rauss A, Laisné MJ, Bonnet F. Infection after transurethral resection of the prostate: variation among centers and correlation with a long-lasting surgical procedure. Association pour la Recherche en AnesthésieRéanimation. Eur Urol. 1998;33(4):365-369. DOI: 10.1159/000019617
4. Guo RQ, Yu W, Meng YS, Zhang K, Xu B, Xiao YX, Wu SL, Pan BN. Correlation of benign prostatic obstruction-related complications with clinical outcomes in patients after transurethral resection of the prostate. Kaohsiung J Med Sci. 2017;33(3):144-151. DOI: 10.1016/j.kjms.2017.01.002
5. Mayer EK, Kroeze SG, Chopra S, Bottle A, Patel A. Examining the 'gold standard': a comparative critical analysis of three consecutive decades of monopolar transurethral resection of the prostate (TURP) outcomes. BJU Int. 2012;110(11):1595-1601. DOI: 10.1111/j.1464-410X.2012.11119.x
6. Osman T, ElSaeed KO, Youssef HA, Shabayek M, Emam A, Hussein MS. Evaluation of the risk factors associated with the development of post-transurethral resection of the prostate persistent bacteriuria. Arab J Urol. 2017;15(3):260-266. DOI: 10.1016/j.aju.2017.05.004
7. El Basri A, Petrolekas A, Cariou G, Doublet JD, Hoznek A, Bruyere F. Clinical significance of routine urinary bacterial culture after transurethral surgery: results of a prospective multicenter study. Urology. 2012;79(3):564-569. DOI: 10.1016/j.urology.2011.11.018
8. Girou E, Rioux C, Brun-Buisson C, Lobel B; Infection Committee of the French Association of Urology. The postoperative bacteriuria score: a new way to predict nosocomial infection after prostate surgery. Infect Control Hosp Epidemiol. 2006;27(8):847-854. DOI: 10.1086/506398
9. Wagenlehner FM, Wagenlehner C, Schinzel S, Naber KG; Working Group "Urological Infections" of German Society of Urology. Prospective, randomized, multicentric, open, comparative study on the efficacy of a prophylactic single dose of 500 mg levofloxacin versus 1920 mg trimethoprim/ sulfamethoxazole versus a control group in patients undergoing TUR of the prostate. Eur Urol. 2005;47(4):549-556. DOI: 10.1016/j.eururo.2005.01.004
10. Kogan M.I., Naboka Yu.L., Ivanov S.N. Risk factors, antibiotic prophylaxis, and treatment of urinary tract infection in transurethral surgery for benign prostatic hyperplasia. Urology Herald. 2022;10(2):99-108. (In Russian). DOI: 10.21886/2308-6424-2022-10-2-99-108
11. Kogan M.I., Naboka Yu.L., Ivanov S.N. Assessment of the infectious factor in transurethral surgery of benign prostate hyperplasia. Urology Herald. 2021;9(3):79-91. (In Russian).
12. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-Specific Antigen-Based Screening for Prostate Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. USA, Rockville: Agency for Healthcare Research and Quality (US); 2018.
13. Arjunlal TS, Deepanjali S, Manikandan R, Medha R. Frequency and clinical significance of prostatic involvement in men with febrile urinary tract infection: a prospective observational study. F1000Res. 2020;9:617. DOI: 10.12688/f1000research.24094.3
14. Ivanov S.N., Kogan M.I., Naboka Y.L., Medvedev V.L. Infectious factor in transuretral surgery of benign prostate hyperplasia: a systematic review and meta-analysis. Urologiia. 2023;(4):141-149. (In Russian). DOI: 10.18565/urology.2023.4.141-149
15. Coric J, Mujic J, Kucukalic E, Ler D. Prostate-Specific Antigen (PSA) and Prostate Volume: Better Predictor of Prostate Cancer for Bosnian and Herzegovina Men. Open Biochem J. 2015;9:34-36. DOI: 10.2174/1874091X01509010034
About the Authors
S. N. IvanovRussian Federation
Sergey N. Ivanov — M.D.; Postgrad. Student, Dept. of Urology, Pediatric Urology and Reproductive Health
Rostov-on-Don
M. I. Kogan
Russian Federation
Mikhail I. Kogan — M.D., Dr.Sc.(Med), Full Prof., Honored Scientist of the Russian Federation; Head, Dept. of Urology, Pediatric Urology and Reproductive Health
Rostov-on-Don
Yu. L. Naboka
Russian Federation
Yulia L. Naboka — M.D., Dr.Sc.(Med), Full Prof.; Head, Dept. of Microbiology and Virology No.1
Rostov-on-Don
V. L. Medvedev
Russian Federation
Vladimir L. Medvedev — M.D., Dr.Sc.(Med), Full Prof.; Head, Dept. of Urology; Deputy CMO for Urology & Head, Urology and Nephrology Centre
Krasnodar
G. A. Palaguta
Russian Federation
Georgy A. Palaguta — M.D.; Assist.Prof., Dept. of Urology, Kuban State Medical University; Urologist, Urology Division No.1
Krasnodar
Review
For citations:
Ivanov S.N., Kogan M.I., Naboka Yu.L., Medvedev V.L., Palaguta G.A. PSA density as a predictor of long-term infectious complications during transurethral resection of the prostate: determining an optimal cut-off value. Urology Herald. 2024;12(1):19-26. https://doi.org/10.21886/2308-6424-2024-12-1-19-26













































