Scroll to:
Pathomorphism of buccal grafts used in surgery of extensive bulbar urethral strictures: immunohistochemical analysis
https://doi.org/10.21886/2308-6424-2023-11-1-80-88
Abstract
Introduction. The adverse effects of urine on unadapted tissues are known. This is also entirely relevant for buccal grafts used in augmentation urethroplasty, where these effects have not been thoroughly studied so far.
Objective. To assess the ongoing pathomorphosis in buccal grafts used for urethral augmentation of extensive strictures in the bulbous region and to evaluate how much urine influences their histological transformation following surgery.
Materials & methods. The study included 15 patients with extensive strictures of the bulbous urethra, who underwent a two-stage augmentation urethroplasty with buccal grafts. The grafts pathomorphosis was studied 6 months after the first surgery stage where urethrotomy had been performed with graft augmentation of the dorsal semicircle and the formation of distal and proximal neomeatuses. Natural urination through the latter was restored on days 14 – 20 following the surgery. During the second stage, six months later, urethral tubularisation was performed with two preliminary biopsies of the proximal and distal segments of the grafts implanted in the areas of the neomeatuses formed earlier. The distal area of the graft had no contact with urine, while this contact has occurred in the proximal segment since restoration of natural urination. In biopsy specimens, pathomorphosis of the grafts was studied using immunohistochemical markers: vimentin, clone SRL33; CD34, clone QBEnd/10; MSA HHF-35; СD3, clone LN10; Bcl-2, clone bcl-2\100\D5; CK-HMW 34BE-12.
Results. It was found that inflammation was minimal in areas of grafts implanted that had no contact with urine, while in areas where such contact occurred it was verified to be pronounced even 6 months after the operation. On the submucosal level, this was manifested by an uneven arrangement of collagen fibers, a dysplastically developed vascular network, uneven proliferation of the endothelium with swelling and loss of cellular connectivity, in contrast to areas where there was no contact with urine. In such areas, the graft submucosa had a dense collagen framework with organized microvasculature and uniform epithelial surface.
Conclusion. The impact of urine on buccal grafts used in augmentation urethroplasty is characterised by the disorganisation of its collagen framework, with a pronounced inflammatory component and the “reactivity” of the epithelial lining to the “toxic agent” that persists even 6 months after surgery. This may underlie the risk of a stenosis relapse in the proximal anastomosis area.
Keywords
For citations:
Mitusov V.V., Voronova O.V., Glukhov V.P., Vasiliev O.N., Amirbekov B.G., Loskutov M.G. Pathomorphism of buccal grafts used in surgery of extensive bulbar urethral strictures: immunohistochemical analysis. Urology Herald. 2023;11(1):80-88. (In Russ.) https://doi.org/10.21886/2308-6424-2023-11-1-80-88
Introduction
Nowadays, the most effective method of treating the extended strictures of the spongiose urethra is augmentation urethroplasty, where fragments of the oral mucosa are used as a graft [1–4].
Currently, there are 5 options for performing such augmentation, which differ in the technique of graft fixation [4].
The frequency of relapses and complications after such operations, regardless of the chosen technique, is 19 – 40%, and the most frequent complications are narrowing in the areas of proximal and distal areas of anastomosis of a graft with a native urethra. In a certain number of cases, contractures occur throughout the graft [5–8].
In recent years, new academic works appeared – their authors noted that the development of such complications may not be based so much on the surgical technique as on the initial morphological structure of the graft itself and the adverse effect of urine on it [9–13].
The study aimed to evaluate the pathomorphosis of buccal grafts used for urethral augmentation due to extended bulbar strictures, and the significance of the effect of urine on their histological transformation after surgery.
Materials and methods
The study included 15 patients with a median age of 42.5 years who underwent two-stage urethroplasty of extended strictures (4 – 7 cm) of the bulbous urethra using buccal grafts.
Stage 1 – urethrotomy with augmentation with a dorsal semicircle graft and the formation of two neomeatus. The urethral catheter was placed in the proximal neomeatus for 14 – 20 days, followed by its removal and restoration of natural urination.
Stage 2 – urethral tubularization, which was performed after 6 months with the preliminary execution of two biopsies from the proximal and distal segments of the engrafted grafts in the areas of the neomeatus formed earlier.
The study of pathomorphosis in transplants was carried out with an assessment of the stromal and vascular components, the degree of inflammation and the reaction of the epithelial layer using the following immunohistochemical markers (IHC).
- Vimentin, a SRL33 clone, to study the stromal component;
- CD34, a clone of QBEnd/10 – to study the vascular component;
- MSA HHF-35 – to study the smooth muscle framework of vessels;
- CD3, a clone LN10 – evaluation of the T-cell component of the inflammatory infiltrate;
- Bcl-2, a clone of bcl-2\100\D5 – evaluation of the B-cell component of the inflammatory infiltrate;
- CK-HMW 34BE-12 – a high-molecular cytokeratin for the study of the epithelial component.
The expression was evaluated by a semi-quantitative method in the following points: 0 – absence, 1 – weak, 2 – moderate, 3 – pronounced reaction of marker-positive cells.
Results
Therefore, 6 months after graft implantation, the expression of the IHC marker Vimentin in its stroma in the proximal meatus area was the highest and was assessed as 3-point, and in the distal meatus area, it was moderate – 2 points (Fig. 1).

Figure 1. Vimentin expression in the graft: A – proximal fragment (3 points), magn. ×200; B – distal fragment (2 points), magn. ×200
These results fully correlate with the vascular component assessment data using CD34 markers (Fig. 2) and MSA (Fig. 3), which made it possible not only to verify the overall vascular structure but also to evaluate the smooth muscle framework in the vessels of the graft stroma. The point values of the differences in the study areas were like those in the stroma of the engrafted grafts – 3 and 2 points.
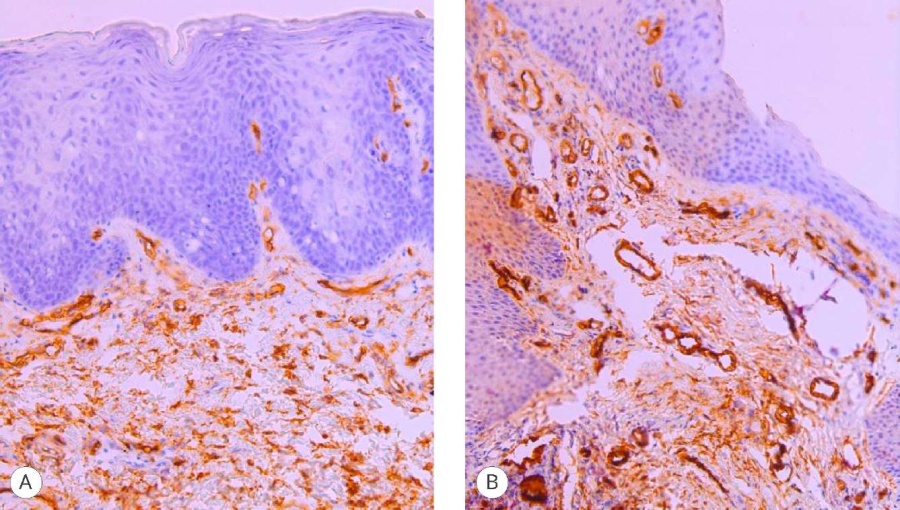
Figure 2. CD 34 expression in the graft: A – proximal fragment (3 points), magn. ×200; B – distal fragment (2 points), magn. ×200
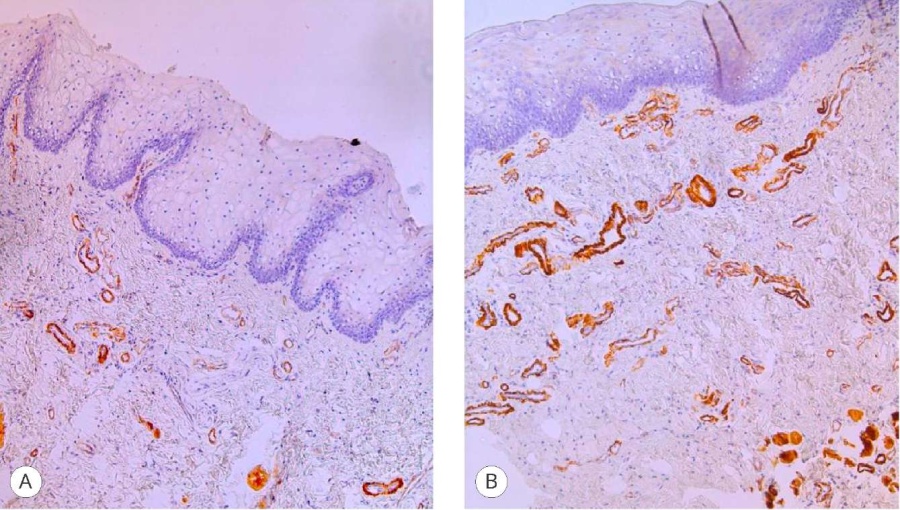
Figure 3. MSA HHF-35 expression in the graft: A – proximal fragment (3 points), magn. ×200; B – distal fragment (2 points), magn. ×200
The most convincing data on the effect of urine on the engraftment of buccal proximal ends of grafts were obtained by studying inflammation after 6 months after their fixation at the first stage of urethroplasty. The study of T-lymphocytes in the inflammatory infiltrate was carried out with a CD3 marker, and B-lymphocytes with a Bcl-2 marker.
In the areas where the graft was not exposed to urine (distal neomeatus), the inflammation was minimal and was rated at 0 points, and in the areas of proximal neomeatus it was characterized as pronounced (3 points) for the T-cell component (Fig. 4) and moderate (2 points) for the B-cell component (Fig. 5).
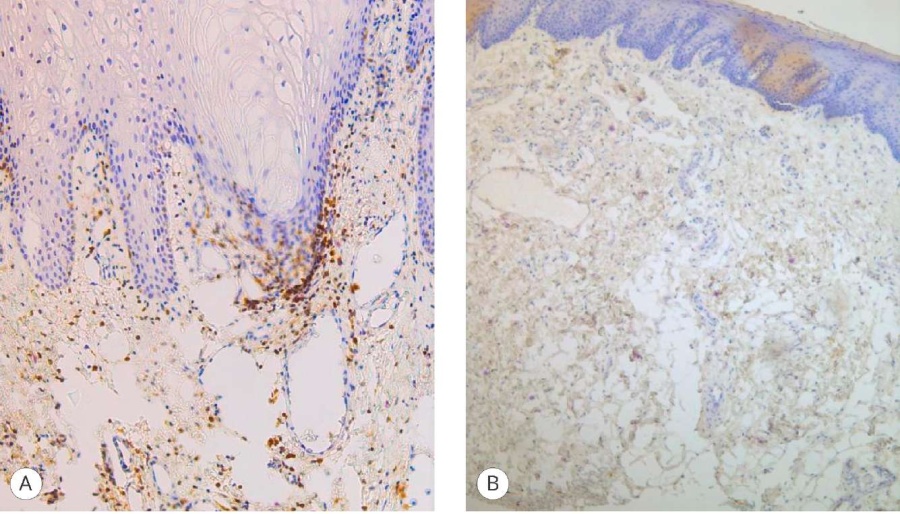
Figure 4. CD3 expression in the graft: A – proximal fragment (3 points), magn. ×100; B) distal fragment (0 points), magn. ×100
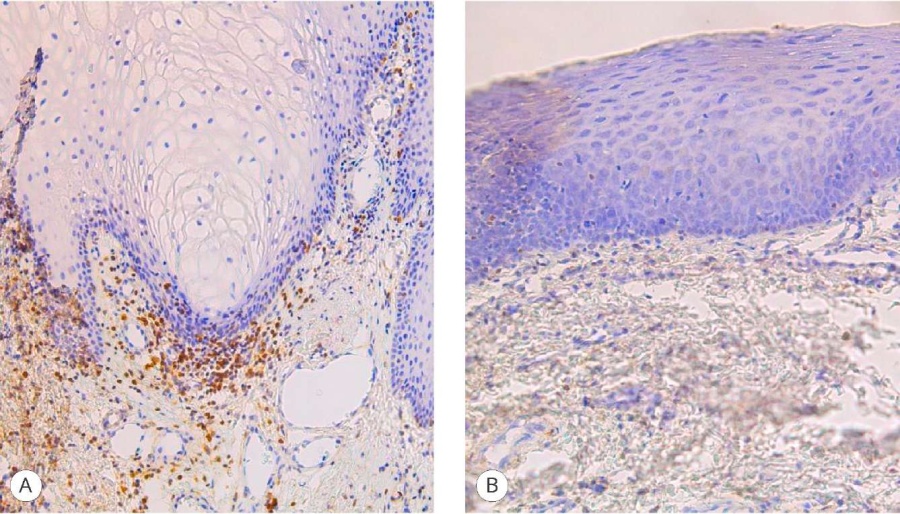
Figure 5. Bcl-2 expression in the graft: A – proximal fragment (2 points), magn. ×200; B – distal fragment (0 points), magn. ×200
Changes in the stroma of the grafts, their vascular structure, and the nature of inflammation when exposed to urine and without it on the grafts could not but affect their epithelial structure. This was fully confirmed not only in the assessment of the marker expression by the IHC scores but also in the visual assessment, which is clearly visible on the microphoto (Fig. 6).
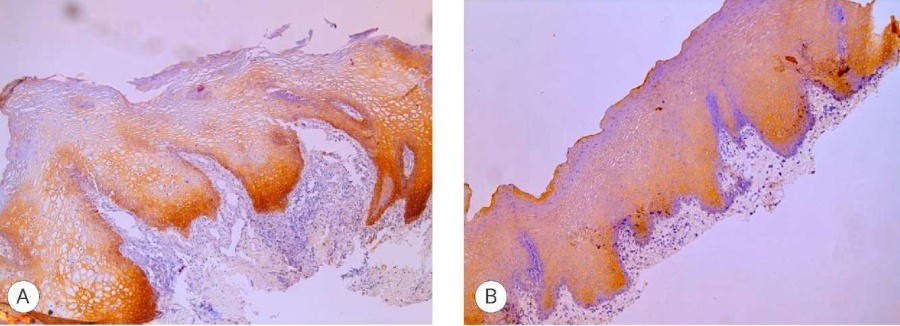
Figure 6. CK-HMW expression in the graft: A – proximal fragment (1 point), magn. ×100; B – distal fragment (3 points), magn. ×100
The IHC marker for assessing changes in the epithelium of transplants was CK-HMW, where its expression in the proximal area was estimated at only 1 point due to pronounced reactive inflammation leading to the epithelial layer stratification, which determined the poor exposure of the marker. In contrast to the distal area, where the expression of the marker reached a value of 3 points due to the absence of inflammation and the presence of a clear uniform structure of the epithelial layer.
Discussion
Augmentation urethroplasty with extended spongy urethral strictures can be performed in one or more stages. It is determined by several factors: etiology, localization, and extent, single or multi-focal foci of obstruction, the degree of inflammation in the spongy body, as well as the personal preference of the surgeon when performing this type of intervention [1–4].
While performing a one-stage intervention, the removal of the urethral catheter is performed on the 14th – 28th day after surgery, and natural urination is restored. From this moment the direct contact of the graft with urine, as a biological medium, takes place in this process.
The significance of the latter, in the authors’ opinion, undoubtedly influences the graft engraftment process, which may be the basis for the subsequent development of a recurrence of urethral narrowing despite the restored patency of the urethra in this stage of disease treatment [6][9–13].
In real clinical practise, it is impossible to 'track' the morphological changes that occur in the graft itself in patients who underwent simultaneous augmentation urethroplasty.
In this study, the authors worked with the ongoing morphological transformation in transplants in patients where two-stage interventions were performed by studying morphological changes in 2 areas in the same patient — around the proximal and distal neomeatus. In the proximal neomeatus, contact with urine occurred 2 – 3 weeks immediately after catheter removal, and in the distal one, it was not present for 6 months before the second stage of the operation – urethral tubularization.
It was found that even 6 months after good graft engraftment according to clinical parameters, which was determined by its appearance, the absence of infiltration sites, scarring and deformation, palpatory 'mobility' and other parameters, histomorphological differences in areas where early contact with urine and without it took place were significant.
Thus, in the areas of transplants where there was no contact with urine (distal neomeatus), inflammation was minimal, and in the areas of proximal neomeatus, it was verified as expressed by T-cell reaction (CD3 marker) and moderate by B-lymphocytes (Bcl-2 marker).
It was extremely important to assess the nature of histological differences in the structure of the submucosal base, which clinically plays the main role in the graft during augmentation urethroplasty, regardless of the technique chosen for its implementation.
Evaluation of the stromal component of the submucosal base in transplants where its contact with urine occurred quite early, even 6 months after surgery, an uneven arrangement of collagen fibres (Vimentin marker) with a dysplastically developed vascular network, uneven endothelial proliferation with swelling, and loss of intercellular connections (CD34 and MSA markers) was revealed. In graft biopsies removed a few centimeters distal, in areas where there was no early contact with urine, the submucosal base had a dense collagen framework with an organised microcirculatory bed, which was proven by the degree of expression of the IHC markers used under microscopy.
The presented data fully correlated with the nature of the detected differences in the epithelial layer of the grafts of the areas studied (marker CKHMW). Thus, the pronounced expression of the IHC marker in the proximal neomeatus area against a discretely weak reaction in the epithelial layer in the distal neomeatus area was indicative, which, undoubtedly, should be regarded as a reaction to the irritating factor, which is urine.
Conclusion
The effect of urine on buccal grafts used in augmentation urethroplasty is characterized by the disorganization of its collagen framework with a pronounced inflammatory component and the “reactivity” of the epithelial lining, which persists even 6 months after the intervention. The revealed differences in the pathomorphosis of engrafted transplants with early exposure to urine and without it can be one of the important determining factors for the risk of disease relapse. At the same time, the risk of relapse is higher when a one-stage urethroplasty is performed than in cases where surgery is performed in two stages of extended strictures of the bulbous urethra.
References
1. Sinelnikov L.M., Protoschak V.V., Shestaev A.Y., Karpushchenko E.G., Yartsev A.A. Urethral stricture: Modern state of the problem (Literature review). Experimental and clinical urology. 2016;2:80-87. (In Russian). eLIBRARY ID: 29899545
2. Horiguchi A. Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: Current topics and reviews. Int J Urol. 2017;24(7):493-503. DOI: 10.1111/iju.13356
3. Barbagli G, Balò S, Montorsi F, Sansalone S, Lazzeri M. History and evolution of the use of oral mucosa for urethral reconstruction. Asian J Urol. 2017;4(2):96-101. DOI: 10.1016/j.ajur.2016.05.006
4. Polyakov N.V., Keshishev N.G., Kazachenko A.V., Trofimchuk A.D., Chernyshev I.V., Darenkov S.P., Apolikhin O.I. The effectiveness off buccal uretrhoplasty for urethral strictures in men: (Review). Experimental and clinical urology. 2019;4:106-113. (In Russian). DOI: 10.29188/2222-8543-2019-11-4-106-113
5. Robine E, Rigaud J, Luyckx F, Le Clerc QC, Madec FX, Bouchot O, Branchereau J. Analyse des taux de succès des urétroplasties pour sténoses de l’urètre bulbaire chez l’homme adulte : revue systématique de la littérature [Analysis of success rates of uretroplasty for adult male bulbar urethral stricture: A systematic review]. Prog Urol. 2017;27(2):49-57. (French). DOI: 10.1016/j.purol.2016.12.003
6. Spilotros M, Sihra N, Malde S, Pakzad MH, Hamid R, Ockrim JL, Greenwell TJ. Buccal mucosal graft urethroplasty in men-risk factors for recurrence and complications: a third referral centre experience in anterior urethroplasty using buccal mucosal graft. Transl Androl Urol. 2017;6(3):510-516. DOI: 10.21037/tau.2017.03.69
7. Mitusov V.V., Kogan M.I., Mirzaev Z.A., Glukhov V.P., Amirbekov B.G. Surgical treatment of extended spongy urethral strictures in men: minimizing the risks of narrowing in the anastomotic zones between the buccal graft and the native urethra using the dorsal inlay technique. Urology Herald. 2021;9(4):60-69. (In Russian). DOI: 10.21886/2308-6424-2021-9-4-60-69
8. Kogan M.I., Glukhov V.P., Ilyash A.V Mitusov V.V., Krasulin V.V., Chibichyan M.B. Complex spongy urethral strictures with multi-stage treatment: predicting the recurrence risk. Experimental and clinical urology. 2022;1:136-141. (In Russian). DOI: 10.29188/2222-8543-2022-15-1-136-141
9. Kogan M.I., Dementieva I.Yu., Mitusov V. V., Glukhov V.P., Krasulin V.V., Sizyakin D.V., Ilyash A.V. Histopathological evaluation of the evolution of oral mucosa grafts used for augmentation urethroplasty. Urology. 2018;5:64-68. (In Russian). DOI: 10.18565/urology.2018.5.64-68
10. Soave A, Steurer S, Dahlem R, Rink M, Reiss P, Fisch M, Engel O. Histopathological characteristics of buccal mucosa transplants in humans after engraftment to the urethra: a prospective study. J Urol. 2014;192(6):1725-9. DOI: 10.1016/j.juro.2014.06.089
11. Cavalcanti AG, Restrepo CF, Simões M, Costa WS, Sampaio FJB, de Souza DB. What Is the Best Way to Prepare A Buccal Mucosa Graft for Urethroplasty? A Histology-Based Preliminary Report. Urol Int. 2018;100(4):397-401. DOI: 10.1159/000488805
12. Bhattar R, Yadav SS, Tomar V. Histopathological changes in oral mucosa in cases of failed augmented urethroplasty. Turk J Urol. 2019;45(3):206-211. DOI: 10.5152/tud.2019.67435
13. Mitusov V.V., Voronova O.V., Kogan M.I., Mirzaev Z.A., Glukhov V.P., Amirbekov B.G. Is there a feasibility of pharmacotherapeutic preparation of the oral mucosa for augmentation urethroplasty? Urology Herald. 2020;8(3):38-46. (In Russian). DOI: 10.21886/2308-6424-2020-8-3-38-46
About the Authors
V. V. MitusovRussian Federation
Valeriy V. Mitusov — M.D., Dr.Sc.(Med), Assoc.Prof.(Docent); Prof., Dept. of Urology, Pediatric Urology and Reproductive Health,
Rostov-on-Don
O. V. Voronova
Russian Federation
Olga V. Voronova ― M.D.; Assist.Prof., Dept. Forensic Medicine,
Rostov-on-Don
V. P. Glukhov
Russian Federation
Vladimir P. Glukhov — M.D., Cand.Sc.(Med), Assoc.Prof. (Docent); Assoc.Prof., Dept. of Urology, Pediatric Urology and Reproductive Health,
Rostov-on-Don
O. N. Vasiliev
Russian Federation
Oleg N. Vasilyev — M.D., Dr.Sc.(Med); Assoc.Prof. (Docent), Dept. of Urology, Pediatric Urology and Reproductive Health,
Head, Urology Division,
Rostov-on-Don
B. G. Amirbekov
Russian Federation
Beikes G. Amirbekov ― M.D., Cand.Sc.(Med); Urologist, Outpatients Clinic,
Rostov-on-Don
M. G. Loskutov
Russian Federation
Micheal G. Loskutov ― Urologist, urological Department,
Rostov-on-Don
Review
For citations:
Mitusov V.V., Voronova O.V., Glukhov V.P., Vasiliev O.N., Amirbekov B.G., Loskutov M.G. Pathomorphism of buccal grafts used in surgery of extensive bulbar urethral strictures: immunohistochemical analysis. Urology Herald. 2023;11(1):80-88. (In Russ.) https://doi.org/10.21886/2308-6424-2023-11-1-80-88













































