Scroll to:
Effect of taxanes on the miR-106 and miR-200c expression in prostate cancer cells in vivo and in vitro
https://doi.org/10.21886/2308-6424-2022-10-4-98-108
Abstract
Introduction. A combination of antiandrogen and cytostatic drugs was justified in the neoadjuvant therapy of patients with high-risk prostate cancer (HiRPCa) in some clinical trials. The effectiveness of such therapy in each individual case depends on the sensitivity of cancer cells to the applied drugs. It makes possible the development of the new technologies to personalize therapeutic approach. MicroRNAs (miRNAs) are a class of regulatory molecules whose expression is altered in PCa cells and can be associated with the sensitivity/resistance of cancer cells to specific cytostatics, for instance, taxanes.
Objective. To identify the potential-marker miRNAs of PCa cells sensitivity to taxanes.
Materials and methods. Samples of PCa tissue (n. 56) obtained from patients underwent neo-adjuvant therapy (antiandrogen and taxanes) and radical prostatectomy; PCa cell lines (PC-3, DU-145, LNCap). Total RNAs isolation was carried out using miRNeasy FFPE Kit, LRU-100-50; miRCURY LNA miRNA Focus PCR Panel, All-MIR kits were used for semi-quantitative analysis of potentially marker microRNA molecules using sequential reverse transcription and PCR.
Results. The effect of taxanes on PCa cells is associated with up-regulation of miR-106b expression and down-regulation of miR-200c expression in both in vivo and in vitro conditions.
Conclusion. MiR-106b and miR-200c miRNAs are involved in the response of PCa cells to taxanes, and therapeutic modification of these molecules in PCa cells may present a potential strategy to increase their sensitivity to taxane-containing therapy. Appropriate innovative technology may be in demand in the treatment of HiRPCa-patients.
For citations:
Plevako D.S., Knyazeva M.S., Sidina E.I., Berkut M.V., Reva S.A., Tolmachev S.S., Artemyeva A.S., Nosov A.K., Malek A.V. Effect of taxanes on the miR-106 and miR-200c expression in prostate cancer cells in vivo and in vitro. Urology Herald. 2022;10(4):98-108. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-98-108
Introduction
Prostate cancer (PCa) occupies a leading position in the statistics of cancer incidence among men [1]. The clinical course of the disease in each case has an individual character, as it is determined by a unique combination of genetic, epigenetic, and morphological features of tumor cells [2]. To optimize the treatment regimen, criteria for the stratification of patients into groups of varying degrees of risk have been developed and are used actively in clinical practice [3]. At the time of disease detection, the high-risk group includes from 10 to 30% of patients for whom active therapeutic tactics are justified. In particular, the implementation of neo-adjuvant therapy can improve the results of radical prostatectomy (RP) in cases of a locally advanced process [4]. The review article by Mikkilineni et al. (2018) presents an analysis of various modes of neo-adjuvant therapy tested in clinical trials [5], but the different design of studies makes it difficult to compare the results and choose the optimal approach. Therefore, the task of developing effective systemic therapy regimens for patients with high-risk PCa (HiRPCa) remains relevant.
During the period from 2014 to 2018, a study was conducted at Petrov National Medical Research Center of Oncology to assess the safety and effectiveness of neo-adjuvant chemohormonal therapy (CHT) performed before radical prostatectomy (RP) in patients suffering from PCa [6]. CHT involved a combination of two medicines: an inhibitor of microtubule depolarization (docetaxel) and an antagonist of gonadotropin-releasing hormone (degarelix). As part of the study, two randomized groups were compared: patients who received a course of neo-adjuvant therapy followed by RP (CHT-RP), and patients who underwent only RP. Patients of the first group tolerated CHT well; they had a lower degree of local tumor prevalence and a greater 3-year overall survival. The authors concluded that the CHT-RP regimen was safe and effective in patients with PCa. However, to further optimize therapeutic approaches in relation to patients with PCa, an in-depth study of the mechanisms of action of the drugs used is necessary. For example, the identification of molecular markers of sensitivity to taxanes and/or molecular targets of modification of this sensitivity may underlie the development of methods for more accurate stratification of patients or technologies to improve the effectiveness of therapy.
MicroRNAs are short regulatory RNA molecules that are involved in the formation of the reaction of tumor cells to the action of cytostatic medicines, including taxanes [7]. For the first time, the participation of a number of microRNA molecules in the formation of resistance of PCa cells to the action of taxanes was shown in 2015 [8]. In a number of subsequent experimental studies, the association of the expression level of a number of molecules (miR-200b-3p, miR-34b-3p and miR-375) with the degree of resistance of PC cells to the action of paclitaxel was shown [9], which indicates the prognostic potential of methods for analyzing these molecules in biopsy material. In addition, therapeutic modification of the concentration of individual molecules (miR-323) is a potential technology for increasing the sensitivity of PCa cells to the effects of docetaxel [10].
The study aimed to search for microRNA molecules whose expression is associated with the reaction of PCa cells to the effects of taxanes during CHT. As part of the work, a comparative analysis of several microRNA molecules was carried out in PCa material from patients who received and did not receive neoadjuvant CHT (CHT-RP vs. RP) was carried out [6], as well as the evaluation of changes in the expression of these molecules in PC cells after exposure to paclitaxel in an in vitro experiment.
Materials and methods
Patients demographics. The study used postoperative material from 56 patients diagnosed with HiRPCa, included in the previously cited study [6]. The CHT-RP group included 32 patients who were treated with docetaxel once every 21 days (75 mg/m2 up to 6 cycles) and a gonadotropin-releasing hormone antagonist degarelix according to the standard scheme (6 subcutaneous injections every 28 days) followed by RP. The RP group included 24 patients who underwent surgical treatment as monotherapy. The clinical characteristics of patients and the results of the evaluation of the effect of therapy (CHT-RP vs. RP) were presented earlier [6]. Sections containing PCa cells (>90%) were prepared from paraffinized samples of surgical material fixed with formalin.
Lines of PCa cells. Three PCa cell lines used in the study were as follows: LNCaP, PC-3, and DU-145. The cells were cultured in RPMI-1640 medium with the addition of 10% embryonic bovine serum and a mixture of Pen-Strep antibiotics, 100 µg/ml (“Biolot, LLC”, St. Petersburg, Russia) under standard conditions. To assess the effect of paclitaxel, cells were grown in a 6-well plate to a confluence of 50–60%, then the standard culture medium was replaced with Paclitaxel medium (Paclitaxel CAS No. 33069-62-4 – “Sigma Aldrich GmbH”, Germany) at concentrations of 10 nM and 20 nM and cultured for 48 hours.
RNA isolation. RNA isolation from paraffin sections was carried out by means of using the miRNeasy FFPE Kit (“Qiagen GmbH”, Hilden, Germany) according to the manufacturer's protocol. RNA isolation from cell lines was carried out using a set of LRU-100-50 reagents (“Biolabmix, LLC”, Novosibirsk, Russia) according to the manufacturer's instructions. The quality of isolated RNA was evaluated using a NanoPhotometr N50 spectrophotometer (“Implen GmbH”, München, Germany), measuring absorption at wavelengths of 260 and 280 nm. The concentration of the isolated RNA was evaluated on a Qubit 2.0 fluorimeter (“Invitrogen Corp.”, Carlsbad, CA, USA).
Evaluation of microRNA expression. The initial analysis of the expression of 84 molecules of oncogenic microRNAs (so-called cancer-associated microRNAs) was carried out by means of using a set of reagents miRCURY LNA miRNA Focus PCR Panel – Human Cancer (“Qiagen GmbH”, Hilden, Germany). Additionally, the authors used the miRCURY LNA RT kit, the miRCURY LNA SYBR ® Green PCR kit (“Qiagen GmbH”, Hilden, Germany) according to the manufacturer's instructions. The expression of individual microRNAs was evaluated using ALL-MIR series kits (“Algimed Techno, LLC”, Minsk, Belarus). All the studies were performed on the CFX96 Sensor device in real time (“Bio-Rad Laboratories, Inc.”, Hercules, CA, USA).
Statistical analysis. The calculation of changes in the expression of individual microRNA molecules was performed by the dCt method (delta cycle threshold), while the average Ct value of all analyzed molecules in a separate sample (the so-called total Ct value) was used as a normalizer according to the formula dCt = 2(total Ct – Ct miR-93). The data were checked for the normality of the distribution using the Kolmogorov-Smirnov and Shapiro-Wilk tests. To assess the statistical significance of the difference in the expression level of individual molecules between the compared groups of patients, calculations of the nonparametric criterion Mann-Whitney U test were performed. To assess the statistical significance of the difference in the expression levels of individual molecules in PCa cells after exposure to paclitaxel, the arithmetic mean values of normalized indicators and the standard deviation were calculated using the formula: STD = √(∑(x – xaverage)2)/n. Data collection, distribution, and analysis were carried out using the software CFX Manager™ (“Bio-Rad Laboratories, Inc.”, Hercules, CA, USA), Microsoft Office Excel 10.0 (“Microsoft Corp.”, Redmond, WA, USA), Sigma Plot 11.0 (“Systat Software, Inc.”, San Jose, CA, USA), and GraphPad Prism 8.0 (“GraphPad Software Inc.”, “Graphpad Holdings, LLC”, San Diego, CA, USA).
Results
Search for regulated microRNA molecules. The study included three stages, which are schematically presented in Figure 1.
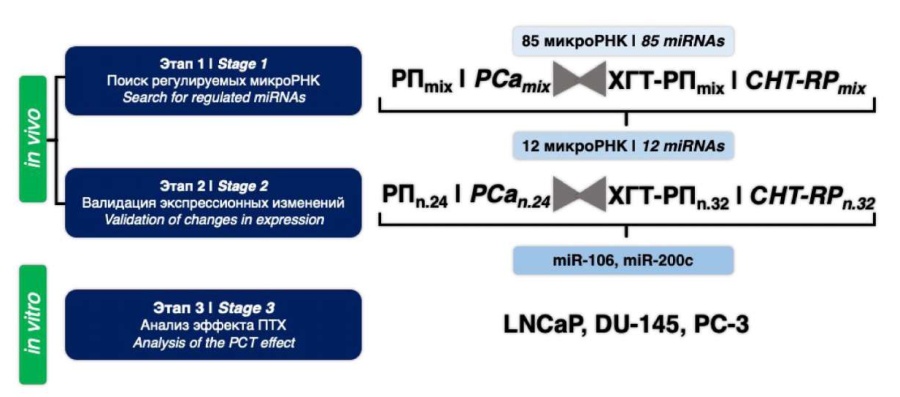
Figure 1. Research design
The task of the first stage was relatively 'wide profiling' of a relatively large number of potentially marker molecules. To do this, the so-called 'RNA pools' (PE and CHT-RP) were prepared from two groups of RNA samples. Each such 'RNA pool' was an equimolar mixture of RNA samples of one comparison group. The total expression profile of 84 microRNA molecules was carried out in each “RNA pool”. Processing of the data obtained on the expression of 84 microRNAs included checking the effectiveness of RNA isolation, inter-tablet calibration, and normalization relative to the arithmetic mean (total Ct). The microRNAs with Ct values greater than 38 were excluded from the analysis. The molecules for which the values of the normalized expression levels for the two “RNA pools” differed less than one and a half times were excluded from the analysis. Thus, 29 molecules were selected. An analysis of scientific literature showed that the relationship between the expression level and the effect of paclitaxel on tumor cells was previously shown for 12 of the 29 selected microRNAs. The list of potentially marker molecules and corresponding sources are presented in the table below.
Table. Potential marker miRNAs
|
miRNAs |
References |
Expression level CHT-RP/RP |
p |
|
16-5p |
0.03 |
0.2 |
0.01 |
|
26b-5p |
0.004 |
0.01 |
0.002 |
|
141-3p |
0.01 |
0.001 |
0.4 |
|
143-3p |
0.001 |
0.02 |
0.003 |
|
145-5p |
0.03 |
0.2 |
0.01 |
|
200b-3p |
0.004 |
0.01 |
0.002 |
|
205-5p |
0.01 |
0.001 |
0.4 |
|
375-3p |
0.001 |
0.02 |
0.003 |
|
451a-5p |
0.03 |
0.2 |
0.01 |
|
27a-3p |
0.004 |
0.01 |
0.002 |
|
106b-5p |
0.01 |
0.001 |
0.4 |
|
200c-3p |
0.001 |
0.02 |
0.003 |
Note. * — arithmetic mean of the normalized values of expression levels of marker molecules for compared CHT-RP and RP groups are presented
Analysis of the expression of potentially marker microRNAs. To validate the differential expression of 12 selected microRNAs in 56 samples of surgical material, the technology of bilateral priming of reverse transcription followed by PCR with two microRNA-specific primers was used (Fig. 1; Stage 2). In this case, the analysis of 12 molecules was carried out for each sample, the results were normalized and the mean values for the compared groups were calculated (Table). Using U test, it was found that the expected differences in microRNA expression between the study groups of patients were observed for all 12 molecules, but these differences had different degrees of statistical significance (Table 1).
Figure 2 demonstrates the results of comparing the relative (normalized) expression of molecules, with the greatest difference between the compared groups. Thus, in most of the cases, the increased expression of the analyzed molecules, including miR-200c, miR-143, miR-375, miR-200b, and miR-27a, was observed in the PCa cells of patients of the CHT-RP group compared with the control group (RP). Only one molecule (miR-106b) showed a statistically significant decrease in expression in the CHT-RP group. Since the regimen of neo-adjuvant therapy of patients in the experimental group included two drugs (docetaxel and degarelix), the results obtained do not allow concluding which drug triggered the change in the expression of microRNA molecules. However, the presented data indicate an obvious effect that the combination of drugs used has on the expression of several microRNA molecules in PCa cells.

Figure 2. Relative expression level of potential marker miRNAs in PCa cells of patients from two groups: neo-adjuvant chemohormonal therapy + radical prostatectomy (CHT-RP) and radical prostatectomy (RP)
Analysis of the effect of paclitaxel on microRNA expression in PCa cells in vitro. The studied CHT scheme [6] suggested a combination of two drugs with different mechanism of action. Docetaxel acted directly on tumor cells, changing the dynamics of microtubule depolymerization and blocking the mitosis process [21]. Degarelix, a selective antagonist of gonadotropin-releasing hormone, competitively bound pituitary receptors, reduced the secretion of gonadotropin hormones by the pituitary gland and, as a consequence, the secretion of testosterone by gonads [22]. Thus, the effect of degarelix was mainly determined by a decrease in the stimulating effect of testosterone on tumor cells, and possible changes in the expression of cellular microRNAs could be the result of this effect. In vitro experimental conditions allow for differentiation of the cytostatic effects of taxanes and testosterone deficiency. Within the framework of this study, the task was to evaluate, which of the changes in microRNA expression observed in vivo were mediated by the action of taxanes. For this purpose, three lines of PCa cells were used: PC-3 and DU145 (androgen-dependent) and LNCaP (androgen-independent) cells. Cell lines were cultured in paclitaxel-containing medium at different concentrations, allowing the dependence of the observed effects on dose. After 48 hours of incubation, the expression of microRNA marker molecules was analyzed. In two cases, changes were observed that were dose-dependent and reproduced in three cell cultures. Thus, paclitaxel activated miR-106b expression, but inhibited miR-200c expression (Fig. 3).
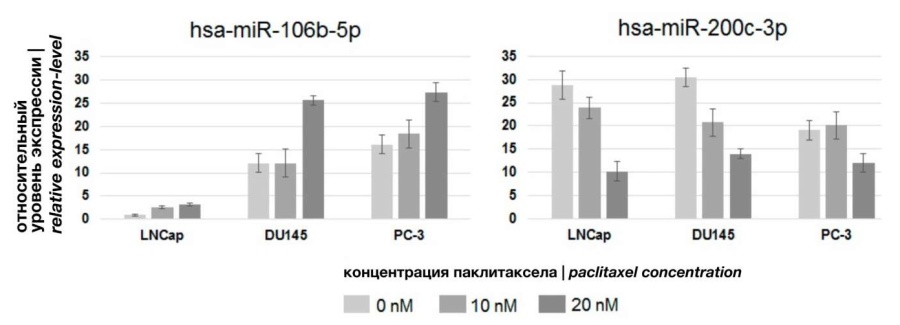
Figure 3. Effect of paclitaxel on miRNAs expression in PCa cells
The recorded changes had a character similar to that observed in the PCa cells of patients after neo-adjuvant HCT. Therefore, exposure to toxins stimulates miR-106b expression activity and inhibits miR-200c expression activity in PCa cells in vivo and in vitro. No changes in the expression activity of other microRNA molecules were observed, which suggests that the change in the expression of these molecules in the tumor cells of patients was the result of drug castration, and not the result of the cytostatic action of docetaxel.
Discussion
Within the framework of the presented study, the task of searching for and studying changes in the expression of microRNA by PCa cells caused by the combined effects of docetaxel and deragelix was solved. The analysis of tumor material of patients who underwent neoadjuvant CHT revealed statistically significant changes in the expression (mainly activation) of several microRNA molecules. The selection of molecules included in the detailed study (Fig. 1; Stage 2) was based on preliminary results of the analysis of 85 tumor-associated microRNAs in 'RNA pools' of images and data from the literature (Fig. 1; Stage 1). Therefore, it is highly likely that many microRNA molecules that “responded” to the effects of CHT remained outside the scope of the study, and the shown changes in the expression of 12 molecules are only a fragment of changes caused by CHT in the microRNA expression profile of PCa cells. Nevertheless, statistically significant shifts in the expression activity of several microRNA molecules confirm the fact of the effect of CHT on the biology of PCa cells. These results are well combined with the data from an earlier assessment of the clinical efficacy of neo-adjuvant CHT.
An interesting phenomenon is the predominantly stimulating effect that CHT had on microRNA expression: of the 12 molecules included in the study, activation of expression was observed in 11 cases, and inhibition of expression in the CHT-RP group was observed only for miR-106b. However, to confirm and correctly interpret this observation, a “broader” analysis is needed, for example, using the “new generation” sequencing technology (NGS, next generation sequencing).
The main result of the final in vitro stage (Fig. 1; Stage 3) of the study was the description of changes in the expression of miR-106b and miR-200c in PCa cells induced by exposure to paclitaxel. Thus, changes in the expression of these molecules in the PCa cells of patients who received a course of HCT and in the cells of three lines (LNCaP, PC-3, DU145) after exposure to paclitaxel had a similar character. This indicates a high probability of participation of miR-106b and miR-200c in the reaction of PCa cells to the effects of taxanes. It is an interesting fact that in a recent review of microRNA studies as therapeutic targets and markers of PCa [23], the Italian authors (Arrighetti and Beretta) also noted the multidirectional nature of the involvement of miR-106b and miR-200c in the development of PCa (Fig. 4).

Figure 4. Scheme of involvement in carcinogenesis and potential for therapeutic modification of miRNAs in PCa cells (adapted from Arrighetti and Beretta [23])
Thus, the 'carcinogenic nature' of miR-106b has been shown in the context of the development of various cancers, including hepatocellular carcinoma [24], breast cancer [25] and PCa. Moreover, in the latter case, the effect of miR-106b was associated with the regulation of adhesive characteristics [25] and the metastatic potential of cells [26]. It can be assumed that the antitumor effect of taxanes in PCa is expressed not only in a direct effect on microtubule dynamics, but is also associated with inhibition of miR-106b expression and a decrease in the malignant potential of PCa cells.
The PubMed electronic library of the American National Institutes of Health (NIH) contains more than 4,000 scientific articles that reflect the role of miR-200c in the development of PCa. These studies describe various mechanisms of miR-200c participation in the regulation of cell biology, but in most cases this molecule is considered an “oncosuppressor”: the prostate guardian miRNA [27]. In this context, activation of miR-200c expression against the background/because of the cytostatic action of taxanes seems to be a natural phenomenon.
In conclusion, it makes sense to note that out of 12 molecules studied in the framework of the first two stages of work, for ten there was no effect of paclitaxel in an in vitro experiment. Therefore, it can be assumed that the reason for the change in the expression of these ten molecules in PCa cells from patients was castration of the drug. Thus, in the context of at least 12 studied molecules, the effect of reducing the stimulating effect of testosterone appears to be more pronounced compared to the effects of docetaxel. It is known that degarelix has a direct effect on PCa cells [28]. This effect affects the mechanisms of regulation of proliferation, cell adhesion, and programmed cell death, and can serve as an explanation for the more significant effect of degarelix (than docetaxel) on the expression status of microRNAs of PCa cells observed in this work. For a more detailed study of this phenomenon, it is necessary to conduct additional in vitro studies and evaluate the isolated effects of degarelix on PCa cell lines.
Conclusion
The scientific search for opportunities and the development of effective and multi-modal therapies for patients with HiRPCa is an unsolved task. This group of patients requires active therapeutic tactics, which can be implemented either by an effective combination of existing methods (chemotherapy, hormone therapy, immunotherapy, radiation therapy, surgery), or by developing and implementing new methods.
MicroRNAs are endogenous regulators of many cellular processes; these molecules are actively involved in the neoplastic transformation of cells and in the reaction of PCa cells to any therapeutic effects [29]. Therapeutic modification of mircoRNA composition in tumor cells is a promising strategy to increase the effectiveness of standard therapies, as, for example, it has been proven for the combination of miR-124 and paclitaxel [30]. Therefore, the study of the participation of these molecules in the formation of a response to HiRPCa therapy may create the basis for the development of innovative therapeutic technologies, especially relevant for this group of patients.
References
1. Kaprin A.D., Starinsky V.V., Shokhzadov A.O., eds. Malignant neoplasms in Russia in 2020 (morbidity and mortality). Moscow: P. Hertsen Moscow Oncology Research Institute – branch of the FSBI «National medical research radiological centre» of the Ministry of Health of Russia; 2021. ISBN 978-5-85502-268-1. (In Russ.) URL: https://glavonco.ru/cancer_register/
2. Cimadamore A, Mazzucchelli R, Lopez-Beltran A, Massari F, Santoni M, Scarpelli M, Cheng L, Montironi R. Prostate Cancer in 2021: Novelties in Prognostic and Therapeutic Biomarker Evaluation. Cancers (Basel). 2021;13(14):3471. https://doi.org/10.3390/cancers13143471
3. Parry MG, Cowling TE, Sujenthiran A, Nossiter J, Berry B, Cathcart P, Aggarwal A, Payne H, van der Meulen J, Clarke NW, Gnanapragasam VJ. Risk stratification for prostate cancer management: value of the Cambridge Prognostic Group classification for assessing treatment allocation. BMC Med. 2020;18(1):114. https://doi.org/10.1186/s12916-020-01588-9
4. Srivatsa N, Nagaraja H, Shweta S, Raghunath SK. Radical Prostatectomy for Locally Advanced Prostate Cancers-Review of Literature. Indian J Surg Oncol. 2017;8(2):175-180. https://doi.org/10.1007/s13193-016-0599-9
5. Mikkilineni N, Hyams ES. Neoadjuvant therapies for surgical management of high-risk, localized prostate cancer. Transl Cancer Res. 2018;7(S6):S662-S675. https://doi.org/10.21037/tcr.2018.05.36
6. Berkut M.V., Artemjeva A.S., Reva S.A., Tolmachev S.S., Petrov S.B., Nosov A.K. Oncological results of neoadjuvant chemohormonal therapy in patients with high and very high-risk prostate cancer. Cancer Urology. 2020;16(1):54-63. (In Russ.) https://doi.org/10.17650/1726-9776-2020-16-1-54-63
7. Cui SY, Wang R, Chen LB. MicroRNAs: key players of taxane resistance and their therapeutic potential in human cancers. J Cell Mol Med. 2013;17(10):1207-17. https://doi.org/10.1111/jcmm.12131
8. Kopczyńska E. Role of microRNAs in the resistance of prostate cancer to docetaxel and paclitaxel. Contemp Oncol (Pozn). 2015;19(6):423-7. https://doi.org/10.5114/wo.2015.56648
9. Samli H, Samli M, Vatansever B, Ardicli S, Aztopal N, Dincel D, Sahin A, Balci F. Paclitaxel resistance and the role of miRNAs in prostate cancer cell lines. World J Urol. 2019;37(6):1117-1126. https://doi.org/10.1007/s00345-018-2501-6
10. Gao Q, Zheng J. microRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73. Biomed Pharmacother. 2018;97:528-534. https://doi.org/10.1016/j.biopha.2017.10.040
11. Huang Y, Chen G, Wang Y, He R, Du J, Jiao X, Tai Q. Inhibition of microRNA-16 facilitates the paclitaxel resistance by targeting IKBKB via NF-κB signaling pathway in hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;503(2):1035-1041. https://doi.org/10.1016/j.bbrc.2018.06.113
12. Zhao B, Zhang J, Chen X, Xu H, Huang B. Mir-26b inhibits growth and resistance to paclitaxel chemotherapy by silencing the CDC6 gene in gastric cancer. Arch Med Sci. 2019;15(2):498-503. https://doi.org/10.5114/aoms.2018.73315
13. Zedan AH, Osther PJS, Assenholt J, Madsen JS, Hansen TF. Circulating miR-141 and miR-375 are associated with treatment outcome in metastatic castration resistant prostate cancer. Sci Rep. 2020;10(1):227. https://doi.org/10.1038/s41598-019-57101-7
14. Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Wang Z, Hua L, Wang X. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350(1-2):207-13. https://doi.org/10.1007/s11010-010-0700-6
15. Fei BY, Wang XY, Fang XD. MicroRNA-143 replenishment re-sensitizes colorectal cancer cells harboring mutant, but not wild-type, KRAS to paclitaxel treatment. Tumour Biol. 2016;37(5):5829-35. https://doi.org/10.1007/s13277-015-4354-6
16. Guan X, Guan Y. miR-145-5p attenuates paclitaxel resistance and suppresses the progression in drug-resistant breast cancer cell lines. Neoplasma. 2020;67(5):972-981. https://doi.org/10.4149/neo_2020_190622N536
17. Yu J, Lu Y, Cui D, Li E, Zhu Y, Zhao Y, Zhao F, Xia S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol Rep. 2014;31(2):910-8. https://doi.org/10.3892/or.2013.2897
18. Puhr M, Hoefer J, Schäfer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol. 2012;181(6):2188-201. https://doi.org/10.1016/j.ajpath.2012.08.011
19. Wang Y, Lieberman R, Pan J, Zhang Q, Du M, Zhang P, Nevalainen M, Kohli M, Shenoy NK, Meng H, You M, Wang L. miR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. Mol Cancer. 2016;15(1):70. https://doi.org/10.1186/s12943-016-0556-9
20. Wang W, Zhang L, Wang Y, Ding Y, Chen T, Wang Y, Wang H, Li Y, Duan K, Chen S, Yang Q, Chen C. Involvement of miR-451 in resistance to paclitaxel by regulating YWHAZ in breast cancer. Cell Death Dis. 2017;8(10):e3071. https://doi.org/10.1038/cddis.2017.460
21. Karahalil B, Yardım-Akaydin S, Nacak Baytas S. An overview of microtubule targeting agents for cancer therapy. Arh Hig Rada Toksikol. 2019;70(3):160-172. https://doi.org/10.2478/aiht-2019-70-3258
22. Doehn C, Sommerauer M, Jocham D. Degarelix and its therapeutic potential in the treatment of prostate cancer. Clin Interv Aging. 2009;4():215-23. https://doi.org/10.2147/cia.s3503
23. Arrighetti N, Beretta GL. miRNAs as Therapeutic Tools and Biomarkers for Prostate Cancer. Pharmaceutics. 2021;13(3):380. https://doi.org/10.3390/pharmaceutics13030380
24. Shi DM, Bian XY, Qin CD, Wu WZ. miR-106b-5p promotes stem cell-like properties of hepatocellular carcinoma cells by targeting PTEN via PI3K/Akt pathway. Onco Targets Ther. 2018;11:571-585. https://doi.org/10.2147/OTT.S152611
25. Wang Z, Li TE, Chen M, Pan JJ, Shen KW. miR-106b-5p contributes to the lung metastasis of breast cancer via targeting CNN1 and regulating Rho/ROCK1 pathway. Aging (Albany NY). 2020;12(2):1867-1887. https://doi.org/10.18632/aging.102719
26. Yin W, Chen J, Wang G, Zhang D. MicroRNA‑106b functions as an oncogene and regulates tumor viability and metastasis by targeting LARP4B in prostate cancer. Mol Med Rep. 2019;20(2):951-958. https://doi.org/10.3892/mmr.2019.10343
27. Andl T, Ganapathy K, Bossan A, Chakrabarti R. MicroRNAs as Guardians of the Prostate: Those Who Stand before Cancer. What Do We Really Know about the Role of microRNAs in Prostate Biology? Int J Mol Sci. 2020;21(13):4796. https://doi.org/10.3390/ijms21134796
28. Sakai M, Martinez-Arguelles DB, Patterson NH, Chaurand P, Papadopoulos V. In search of the molecular mechanisms mediating the inhibitory effect of the GnRH antagonist degarelix on human prostate cell growth. PLoS One. 2015;10(3):e0120670. https://doi.org/10.1371/journal.pone.0120670
29. Doldi V, El Bezawy R, Zaffaroni N. MicroRNAs as Epigenetic Determinants of Treatment Response and Potential Therapeutic Targets in Prostate Cancer. Cancers (Basel). 2021;13(10):2380. https://doi.org/10.3390/cancers13102380
30. Chen C, Shen M, Liao H, Guo Q, Fu H, Yu J, Duan Y. A paclitaxel and microRNA-124 coloaded stepped cleavable nanosystem against triple negative breast cancer. J Nanobiotechnology. 2021;19(1):55. https://doi.org/10.1186/s12951-021-00800-z
About the Authors
D. S. PlevakoRussian Federation
Daniil S. Plevako — Postgrad. Student, Research Laboratory Assistant, Laboratory of Subcellular Technologies, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
M. S. Knyazeva
Russian Federation
Margarita S. Knyazeva — Junior Researcher, Laboratory of Subcellular Technologies, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
E. I. Sidina
Russian Federation
Elena I. Sidina — Junior Researcher, Laboratory of Subcellular Technologies with the Endocrine Oncology Unit, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
M. V. Berkut
Russian Federation
Maria V. Berkut — M.D., Cand.Sc.(Med), Urologist, Oncological Urology Division, Petrov National Medical Research Institute of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
S. A. Reva
Russian Federation
Sergey A. Reva — M.D., Cand.Sc.(Med), Researcher, Petrov National Medical Research Center of Oncology; Head, Oncology Division No. 6 (Andrology and Oncological Urology), Research Center of Urology, Pavlov First Saint-Petersburg State Medical University (Pavlov University).
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
S. S. Tolmachev
Russian Federation
Stanislav S. Tolmachev — M.D., Pathologist, Pathology Division, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
A. S. Artemyeva
Russian Federation
Anna S. Artemyeva — M.D., Cand.Sc.(Med), Head, Research Laboratory of Tumor Morphology, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
A. K. Nosov
Russian Federation
Aleksander K. Nosov — M.D., Cand.Sc.(Med), Head, Oncological Urology Division, Senior Researcher, Dept. of General Oncology and Urology, Assoc.Prof., Methodological Accreditation and Simulation Centre, Petrov National Medical Research Institute of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
A. V. Malek
Russian Federation
Anastasia V. Malek — M.D., Dr.Sc.(Med), Head, Laboratory of Subcellular Technologies with Endocrine Oncology Unit, Petrov National Medical Research Center of Oncology.
68 Leningradskaya St., Pesochniy, St.Petersburg 197758
Competing Interests:
The authors declare no conflicts of interest
Review
For citations:
Plevako D.S., Knyazeva M.S., Sidina E.I., Berkut M.V., Reva S.A., Tolmachev S.S., Artemyeva A.S., Nosov A.K., Malek A.V. Effect of taxanes on the miR-106 and miR-200c expression in prostate cancer cells in vivo and in vitro. Urology Herald. 2022;10(4):98-108. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-98-108













































