Scroll to:
Influence of vesicourethral segment reconstruction techniques in radical prostatectomy on urinary continence: evaluation of immediate and long-term outcomes
https://doi.org/10.21886/2308-6424-2022-10-4-54-69
Abstract
Introduction. Currently, various methods and modifications of radical prostatectomy (RP) have been developed and tested, aimed at preventing and minimizing the development of urinary incontinence (UI). However, UI remains an urgent problem in patients who undergo RP, especially at the early follow-up stages.
Objective. To evaluate and compare the effectiveness of modified reconstructive techniques for vesicourethral anastomosis in radical prostatectomy for the prevention of urinary incontinence with respect to the standard technique at different follow-up periods.
Materials and methods. Design: single-centre, clinical, simple, comparative, parallel-group study with retrospective and prospective material evaluation, conducted in 2017 – 2022. Patients: men with verified prostate cancer cT1a – 2cN0 – xM0 without decompensated comorbidities. Age: 45 – 78 years. Retrospective part — group (G) 1: 90 patients who underwent non-nerve-sparing open retropubic RP with a "classic" vesicourethral anastomosis (VUA). Prospective part — G2: 46 patients who underwent similar surgery with modified VUA in two variations: without and with prostatic urethra-sparing — G2a (n = 25) and G2b (n = 21), respectively. Initial examination: standard preoperative laboratory and instrumental examination, assessment of lower urinary tract symptoms (LUTS) using the IPSS-QoL questionnaire. Follow-up examination: objective evaluation of UI according to established criteria and subjective assessment using the ICIQ-SF questionnaire, tracking the dynamics of LUTS using IPSS-QoL. Follow-up periods: 0-point (after catheter removal), 1, 3, 6, and 12 months (exit-point); the dynamics of recovery of urinary continence (UC) was determined monthly. Statistical analysis: Statistica ver.10.0 (StatSoft Inc., Tulsa, OK, USA) using non-parametric methods (CL p < 0.05 at a = 0.05)
Results. Preoperative demographic, questionnaire and instrumental statistics did not differ (p > 0.05) between the groups, confirming the homogeneity of the samples. After RP, the urethral catheter was removed in a period of 7 to 21 days. There was no difference (p > 0.05) in the duration of drainage between the groups. Total urinary continence (TUC) immediately after catheter removal was detected in G1, G2a and G2b in 20.0%, 44.0% and 57.1% of cases, respectively. Subsequent objective monitoring of UC recovery from 1 month showed differences (p < 0.001) between the groups in the dynamics of rehabilitation during the year. The improvement in UC over the one-year follow-up period was cumulatively achieved in G1, G2a and G2b in 48.9%, 44.0% and 33.3% of cases, respectively. Total UI persisted in G1 and G2a by month 12 in 22.2% and 8.0% of patients, respectively, and was not detected in G2b. The severity of UI by the end of the follow-up according to the ICIQ-SF data was the most pronounced (p < 0.001) in patients from G1. TUC-patients in all groups from 1 month showed a marked decrease in the severity of obstructive and irritative LUTS and improved quality of life, with no differences (0.157 < p < 0.390) in IPSS-QoL values between groups.
Conclusions. The use of modified VUA reconstruction techniques made it possible, compared with the standard one, to achieve high continence rates in patients both immediately after the removal of the urethral catheter and at subsequent follow-up periods, without the formation of severe iatrogenic obstruction. Prostatic urethra-sparing modification is the most effective technique that provided the rehabilitation of UI to a complete and/or social level in all patients within a year after surgery.
Keywords
For citations:
Kogan M.I., Belousov I.I., Mitusov V.V., Tokhtamishyan S.K., Ismailov R.S. Influence of vesicourethral segment reconstruction techniques in radical prostatectomy on urinary continence: evaluation of immediate and long-term outcomes. Urology Herald. 2022;10(4):54-69. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-54-69
Introduction
Despite the development of minimally invasive and non-invasive technologies for the treatment of prostate cancer (PCa), radical prostatectomy (RP) remains the preferred therapeutic modality both for local PCa with any risk of progression and in selected patients with a locally advanced process with/without invasion to lymph nodes (cN1+) [1–3]. This is due to the preservation of a leading position in oncological outcomes for patients with PCa who have undergone RP, compared to other main therapeutic approaches — active surveillance / watchful waiting and external beam radiation therapy. The most authoritative multicenter randomized clinical studies SPCG-4 (2018, 23 years of follow-up), PIVOT (2017, 19.5 years of follow-up), and ProtecT (2016, 10 years of follow-up) demonstrated that in patients with low- and intermediate-risk PCa after RP, cancer-specific survival was 80.4%, 91.5 – 95.9%, and 99.0%, respectively [4–6].
Nevertheless, despite the high oncological indicators of the effectiveness of radical surgical treatment, at the present stage of patient-centered medicine, the functional results of RP also play a significant role, affecting the postoperative quality of life (QoL), especially in groups of men with an expected lifetime of more than 10 years [7][8]. It is believed that the factors determining the QoL today are continence without the possibility of using adsorbed underwear (pads-free) and the ability to achieve an erection (with or without the use of phosphodiesterase type 5 inhibitors), sufficient for full-fledged coitus [9][10]. At the same time, only 37.0% of men after RP (n = 182) aged 61 – 88 years (the age of the most frequently performed RP for PCa treatment) reported distress and depression associated with loss of erection [11]. In addition to these data, Salonia et al. (2008) found that no more than 50% of men after this type of surgery were ready to undergo rehabilitation therapy to restore erectile function [12]. In part, according to the results of the Massachusetts Male Aging Study (1994) and the European Male Aging Study (2010), this may be due to sufficiently high rates of moderate and severe preoperative erectile dysfunction, prevailing in the age group of 60 – 69 years [13][14]. Thus, patients with erectile dysfunction at the initial level may be psychologically more prepared for the lack of adequate copulatory function in the postoperative period.
In turn, urinary incontinence (UI) has a more significant impact on the social maladaptation of patients who have undergone RP, due to the presence of constant leakage or uncontrolled loss of urine. According to the report of the 6th International Consultation on Incontinence (2018), from 1.0% to 40.0% of patients have persistent UI after RP [15]. According to questionnaires, up to 80.6% of patients after radical surgery identified their condition associated with UI as “severe” or “very severe” [16].
It should be noted that the introduction of new technical approaches to the implementation of RP (laparoscopy, robotic surgery) did not significantly increase the effectiveness in improving the functional outcomes of postoperative urinary continence (UC) [17][18]. Undoubtedly, the introduction of robotic surgery made it possible to obtain a highly detailed view of the surgical field, dose the energy-mechanical effect on tissues, use modern methods of intraoperative navigation, and perform tissue dissection more precisely to maximize the preservation of other structures (external urethral sphincter, detrusor and ureterotrigonal muscle complex, muscle-fascial structures of the pelvic floor) that ensure the functioning of the sphincter mechanism, after removal of the prostate. However, after RP, the functional activity of the internal urethral sphincter is disrupted; so, the main mechanism of UI is preserved mainly due to the activity of the external urethral sphincter and the musculofascial components of the pelvic floor, which are also subject to certain trauma during surgery [19][20].
At the present stage, a significant number of reconstructive techniques have been developed that complement the standard technique of performing RP and are aimed at both maximum possible preservation and restoration of anatomical structures that provide the main structural mechanism in the postoperative period. These techniques have differences in the approaches used, surgical techniques, and “anatomical landmarks” [21–24]. Despite this, an analysis of existing publications in this area has shown that the use of these techniques allows achieving significant results (> 90.0% of observations) with respect to the complete restoration of continence only in late follow-up periods (6–12 months or more). The problem of total UI, which occurs immediately after the removal of the urethral catheter and persists during the six-month follow-up period, remains relevant. In this regard, the current search aims to develop and implement an optimal reconstructive method that ensures not only the actual restoration of the UC function but also, equally importantly, its restoration at the earliest possible time after surgical treatment.
The study is aimed to evaluate and compare the effectiveness of modified reconstructive techniques for the formation of vesicourethral anastomosis during radical prostatectomy for the prevention of urinary incontinence related to the standard technique at different follow-up periods.
Materials and methods
Study design: a single-center clinical simple comparative study in parallel groups with retrospective and prospective evaluation of the material, performed in the period from 2017 to 2022. Ethical statement: the study was planned and developed in accordance with the provisions of the Helsinki Declaration (Declaration of Helsinki, revised in Fortaleza, Brazil, October 2013) and the principles of Good Clinical Practice (GCP Guidelines), approved by the Ethics Committee of the Rostov State Medical University based on the submitted design (Protocol No. 17/17, approved on October 12, 2017).
Patient demographics. Patients: men with biopsy-verified PCa at cT1a-2cN0-xM0 stages without decompensated comorbidities. Age: 45–78 years. All the patients underwent medical examination, in-patient treatment, and follow-up based on the Urology Division, the Rostov State Medical University Clinic. The retrospective part included 90 patients (group 1) who underwent a non-surgical technique of open retropubic RP (orRP) using the technique of vesicourethral anastomosis (VUA) according to the “classical” method of P.C. Walsh (1983) [25]. The prospective part included 46 patients (group 2) with a similar orRP technique, but with a different type of anastomosis: group 2a (n = 25) — precision isolation and bladder neck (BN)-sparing following anastomosis with a stump of the membranous urethra (MU); group 2b (n = 21) — along with sparing, at least 2 cm of the proximal prostatic urethra was also allocated to the BN. In this case, a urethro-urethroanastomosis 'end-to-end' was formed (between the proximal prostatic urethra and the stump of the MU) [26].
Initial examination. At the prehospital stage, patients underwent a standard preoperative laboratory and instrumental medical examination, necessary and sufficient for this type of surgery, assessment of the severity of lower urinary tract symptoms (LUTS) using the International Prostate Symptom Score – Quality of Life (IPSS-QoL) questionnaire. Regarding nocturnal urination, it was considered that the patient suffered from nocturia more than in one case, if the number of daily urinations exceeded 8; this was considered as a hyperactive bladder. The main demographic indicators of patients in groups at the preoperative stage are shown in Table 1.
Table 1. Initial demographics of patients in comparison groups
|
Demographics |
Retrospective part |
Prospective part |
||
|
Group 1 |
Group 2a |
Group 2b |
||
|
Age, years |
min — max |
48.0 – 78.0 |
45.0 – 76.0 |
49.0 – 73.0 |
|
BMI, kg/m2
|
19.4 – 34.7 |
22.6 – 34.6 |
20.6 – 37.4 |
|
|
Prostate volume, cm3
|
16.0 – 133.0 |
20.4 – 81.5 |
16.2 – 86.0 |
|
|
Post-void residual volume, ml |
0.0 – 250.0 |
0.0 – 269.0 |
0.0 – 260.0 |
|
|
LUTS, points |
4.0 – 32.0 |
6.0 – 29.0 |
4.0 – 31.0 |
|
Note. BMI — body mass index; LUTS — lower urinary tract symptoms
Observational examination. In the postoperative period, the established criteria for comparing the outcomes of surgery for UI were evaluated using the questionnaire International Conférence on Incontinence Questionnaire – Short Form (ICIQ-SF), and the dynamics of LUTS were evaluated using IPSS-QoL. The control follow-up periods (control checkpoints) for the questionnaire were the 'zero point' (after catheter removal), 1, 3, 6, and 12 months. The dynamics of the restoration of UC function was determined monthly. In the postoperative period, all the patients performed exercises according to the Kegel method, according to the brochures issued with a description of the technique. The criteria for the comparative assessment of UI were established: 1) UC immediately after removal of the urethral catheter; 2) the restoration rate of UC during 12 follow-up months; 3) assessment of the UI degree during 12 follow-up months; 4) the function of UC after 12 follow-up months. Also, the criteria for assessing the outcomes of postoperative UC after removal of the urethral catheter, starting from the second day onwards, were determined: 1) total urine continence (TUC) — defined as the absence of urine loss; 2) partial urine continence (PUC) — defined as the ability to retain urine in the body position “supine position” and the ability to perform an arbitrary act of urination when changing the position of the body, requiring periodic use of absorbent underwear (social continence); 3) total urinary incontinence (TUI) — defined as the lack of control over urination, requiring full-time use of absorbent underwear.
Statistical analysis. Statistical processing of the data obtained and hypothesis testing were carried out using the Statistica ver.10.0 software (“StatSoft Inc.”, Tulsa, OK, USA). Shapiro-Wilk and Kolmogorov-Smirnov tests showed no normal distribution of values. Consequently, descriptive statistics of indicators were calculated in the form of the median (Me) and interquartile range (25 and 75 quartiles) and presented as (Me [ Q1; Q3]), as well as descriptions of the minimum – maximum of indicators (min – max). The comparison of indicators in independent samples was performed using a non-parametric statistical method — one-way ANOVA Kruskal-Wallis H test with Dunn's post-hoc test and Pearson's chi-squared test adjusted for likelihood ratio. The accepted confidence level is p < 0.05 at a = 0.05.
Results
Evaluation of preoperative data. A comparative analysis of demographic, questionnaire, and instrumental data revealed no differences (H test = 0.103 < p < 0.899) in the corresponding baseline indicators between patient groups (Table 2), indicating the absence of confounding. It should be noted that the groups were relatively homogeneous in the severity of the LUTS, both according to the cumulative indicators of the IPSS questionnaire and with a separate analysis of obstructive and irritative symptoms. This fact was confirmed by almost identical LUTS-mediated QoL indices in the comparison groups.
Table 2. Baseline indicators of comparative analysis
|
Indicators |
Retrospective part |
Prospective part |
р |
||
|
Group 1 |
Group 2a |
Group 2b |
|||
|
Demographics, Me [ Q1; Q3] (min-max) |
|||||
|
Age, years |
62.0 |
64.0 |
64.0 |
0.426 |
|
|
Age timelines, % |
Mature |
32.2 |
20.0 |
28.6 |
|
|
Elderly |
65.6 |
68.0 |
71.4 |
||
|
Senile |
2.2 |
12.0 |
- |
||
|
BMI, kg/m2
|
27.5 |
28.0 |
28.5 |
0.474 |
|
|
Questionnaire, Me [ Q1; Q3] (min-max) |
|||||
|
LUTS, points |
12.5 |
13.0 |
15.0 |
0.564 |
|
|
Obstructive LUTS, points |
7.0 |
6.0 |
6.0 |
0.652 |
|
|
Irritative LUTS, points |
6.0 |
7.0 |
10.0 |
0.103 |
|
|
Quality of life index, points |
3.5 |
3.5 |
3.0 |
0.899 |
|
|
Instrumental data, Me [ Q1; Q3] (min-max) |
|||||
|
Prostate volume, cm3
|
45.9 |
41.1 |
37.0 |
0.227 |
|
|
Post-void residual volume, ml |
49.0 |
60.5 |
39.0 |
0.412 |
|
|
Patients with PVR >50 ml, % |
22.2 |
27.2 |
14.3 |
||
|
Patients with nocturia, % |
28.9 |
31.8 |
28.6 |
||
|
Patients with OAB, % |
28.9 |
27.3 |
23.8 |
||
Notes: 1. Me — median; Q1 — lower quartile; Q3 — upper quartile; BMI — body mass index; PVR — post-void residual volume; OAB — overactive bladder syndrome; LUTS — lower urinary tract symptoms. 2. p — Kruskal-Wallis H test with Dunn's post-hoc test
Evaluation of postoperative data on control follow-up periods. Assessment of the urinary incontinence severity. In the postoperative period, the duration of bladder urethral drainage in the groups ranged from 7 to 21 days. There were no differences (p = 0.426) between the groups at the time of removal of the urethral catheter. After restoring self-urination, TUI was most pronounced in group 1 — 61.1% of the patients observed an inability to retain urine after catheter removal; in contrast, the most favorable results for UC were determined in group 2b — only 19.1% of the men reported an inability to control urine loss (Table 3).
Table 3. Evaluation of continence function after removal of the urethral catheter
|
Characteristics |
Retrospective part |
Prospective part |
p |
|
|
Group 1 |
Group 2a |
Group 2b |
||
|
Removal of the urethral catheter, days |
12.5 |
10.0 |
9.0 |
0.426 |
|
Total urine continence, % |
20.0 |
44.0 |
57.1 |
|
|
Partial urine continence, % |
18.9 |
32.0 |
23.8 |
|
|
Total urine incontinence, % |
61.1 |
24.0 |
19.1 |
|
Notes: 1. Me — median; Q1 — lower quartile; Q3 — upper quartile. 2. p — Kruskal-Wallis H test with Dunn's post-hoc test
Subsequent objective monitoring of restoration of UC from 1 follow-up month showed the presence of differences (H test = p < 0.001) between groups in the dynamics of rehabilitation during the year. In group 1, the rehabilitation of the UC was noted only from the fourth month, and the peak occurred in the 12 follow-up month. On the contrary, in groups 2a and 2b, UC rehabilitation was already recorded from the 1 follow-up month (an increase of +42.9% and +27.3% of patients), and by the 9 and 7 follow-up months, respectively, the dynamics of UC recovery in the groups showed a minimal increase (Fig. 1). Thus, improvement of UC over the annual follow-up period in groups 1, 2a, and 2b was achieved in 48.9%, 44.0%, and 33.3% of cases, respectively. As a result, at 12 follow-up months, in groups 1 and 2a, the proportion of patients with TUC, PUC and TUI was 22.2%, 46.7%, 31.1% and 56.0%, 36.0%, 8.0%, respectively; In turn, no patients with TUI were identified in group 2b, and the ratio of TUC respondents to PUR was determined at the level of 66.7% to 33.3%.
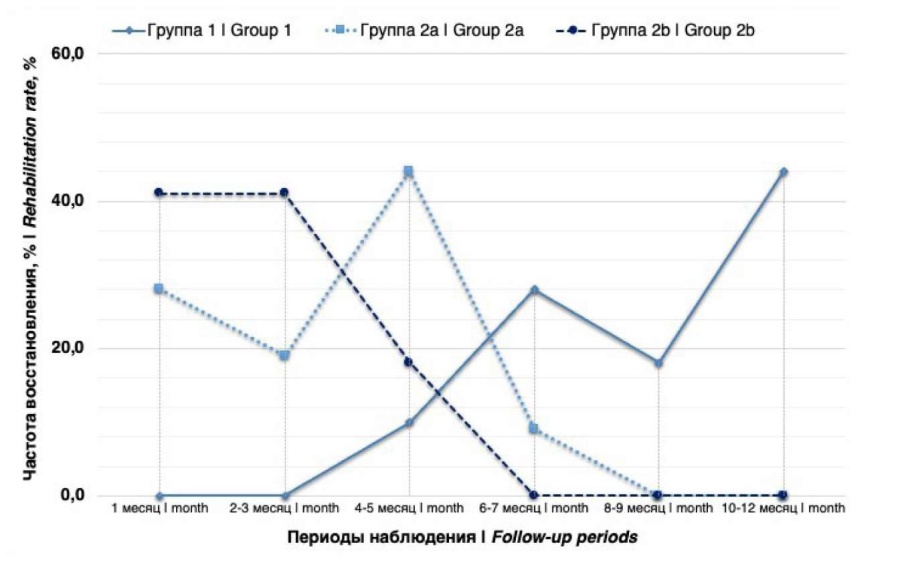
Figure 1. Dynamics of urinary сontinence function rehabilitation in groups during the follow-up period
The completeness of this analysis is determined by assessing the severity of incontinence, demonstrating the quantitative and qualitative components of surgically-mediated UI.
Analysis of the dynamics of changes in the indicators of the ICIQ-SF questionnaire characterizing the migration UI severity also showed the presence of significant differences between the groups in the control checkpoints. The highest median score associated with the severity of UI was noted by patients of group 1. This indicator in the group changed slightly in the control checkpoints, according to the survey data presented later. In groups 2a and 2b, relative to group 1, significantly (p < 0.05) lower score indicators for this criterion were observed, both in the first control checkpoint and dynamics throughout the follow-up period. However, in group 2a, the regression of the score indicators that characterize the migration of the UI severity from the first to the twelfth follow-up month was the most significant among the comparison groups (Table 4).
Table 4. Evaluation of the migration trends of incontinence grades in groups during the follow-up period: analysis of quantitative ICIQ-SF indicators
|
Characteristics |
Retrospective part |
Prospective part |
p |
||
|
Group 1 |
Group 2a |
Group 1 |
|||
|
UI grade, 1st month, points |
Me [ Q1; Q3] (min — max) |
20.0 |
17.5 |
19.0 |
0.005 |
|
UI grade, 3rd month, points |
19.0 |
16.0 |
18.0 |
0.000 |
|
|
UI grade, 6th month, points |
19.0 |
11.0 |
14.0 |
0.000 |
|
|
UI grade, 12th month, points |
17.0 |
10.0 |
11.0 |
0.001 |
|
Notes: 1. Me — median; Q1 — lower quartile; Q3 — upper quartile; UI — urinary incontinence. 2. p — Kruskal-Wallis H test with Dunn's post-hoc test
The analysis of the ratio of grade of UI severity during the restoration of the continence function throughout the follow-up year also showed significant differences in the groups. In group 1, the proportion of patients who achieved complete control of urination did not change significantly during the entire follow-up period; the most significant change over 12 months was recorded in a decrease in the proportion of patients with “very severe” UI, but in turn, the proportion of patients who reported “severe” UI was dominant by this follow-up period. On the contrary, in groups 2a and 2b, both initially after the removal of the urethral catheter and a year later, patients with TUC prevailed, and patients with a “very severe” UI grade were not detected; in turn, by the 12 follow-up month, the proportion of continent patients in group 2b was the most significant among the comparison groups, along with the lowest frequency preservation of “severe” UI (Fig. 2).

Figure 2. Evaluation of the migration trend of incontinence grades in groups during the follow-up period: frequency analysis of indicators
Assessment of lower urinary tract symptoms. It was carried out only in patients in groups with preserved the independent urination, i.e. meeting the criteria of TUC and PUC. It should be noted that the focus of the IPSS-QoL questionnaire, which was not validated to assess any UI parameters and the corresponding effect of UI on the QoL, was explained to patients with PUC before the questionnaire. A comparative analysis of the severity of LUTS in the groups showed the presence of positive dynamics, reflected in a decrease in the indicators of the IPSS-QoL questionnaire from the first follow-up month. In all groups, there was a significant synchronous reduction of LUTS severity caused by both irritative and obstructive symptoms. It should be noted that no differences were found between the groups in any of the control checkpoints (H test = 0.157 < p < 0.390) in indicators characterizing obstructive LUTS. This fact indicates that, regardless of the method used for the formation of VUA, the patients in the groups had no signs of infravesical obstruction (IVO) (Fig. 3).

Figure 3. Evaluation of the dynamics of lower urinary tract symptoms (LUTS) and quality of life (QoL) in groups during the follow-up period
Also, the groups recorded an associated improvement in the QoL from the first follow-up month related to the preoperative level, mediated by a significant decrease in the severity of LUTS. The median indicators of the patients' QoL index corresponded to a “good” level (1–2 QoL points) and had no significant intergroup differences (H test = 0.190 < p < 0.850) in the established control checkpoints (Fig. 3).
Discussion
During this study, objective and questionnaire indicators were compared according to established criteria characterizing the function of postoperative UC in certain follow-up periods in two groups of patients who underwent orRP with VUA using standard (group 1) and modified (group 2) techniques. In the prospective group of modified technique, reconstruction was carried out in two technically similar ways. The main feature of these techniques was the precision of dissection and isolation, as well as the preservation of anatomical structures that are part of the external (i.e. MU) and internal (i.e. BN) urethral sphincter. However, in group 2b, a distinctive feature of the technique was to isolate and preserve as much as possible (but not more than 2 cm, to avoid atrophic changes at the urethral end) the proximal part of the prostatic urethra up to the LUTS, without neglecting the oncological principles mentioned above. The nerve-sparing RP technique was not used in any of the groups, and therefore the formation of UI of varying severity in a certain number of patients was predicted in the postoperative period. Analysis of the main initial preoperative indicators (age, body mass index, prostate volume, and severity of LUTS) did not reveal statistically significant differences between the groups. The focus on the evaluation of these indicators was important since the severity of the initial symptoms has a certain effect on the subsequent functional status of the lower urinary tract in the postoperative period.
Immediately after the removal of the urethral catheter, patients with control over urine leakage were identified in all groups. The proportion of TUC-patients in groups 2a, 2b significantly exceeded vs group 1 and amounted to 44.0%, 57.1% vs 20.0%, respectively. The proportion of patients with PUC also appeared to prevail in groups 2a, 2b compared to group 1 and was 32.0%, 23.8% vs 18.9%, respectively — this condition can also be considered a relatively successful functional result of surgery due to the greater chance of subsequent rehabilitation to the TUC level during the long-term follow-up. Thus, at the follow-up “zero point”, in total, TUC + PUC patients in group 2b were noted in 80.9% of cases, in group 2a — in 76.0% of cases, in group 1 — only in 38.9% of cases. During the subsequent follow-up period, the continence function was restored in patients with significantly different dynamics: in groups 2a and 2b, significant continence rehabilitation was noted already from the first month, and in group 1 — from the seventh month; at the same time, in the group of standard technique, maximum rehabilitation was noted only by the twelfth month, and the use of modified techniques reduced the recovery period to 6 – 8 months.
Thus, from the 6 follow-up month, there were no patients with TUI in group 2b, and only 9.5% of PUC-patients (33.0%) by the end of the follow-up period regarded their condition as “severe” according to the survey data. In turn, in group 2a, by the 12 follow-up month , TUI and PUR persisted in 8.0% and 36.0% of patients, respectively, but at the same time, none of the respondents assessed their condition as “very severe” from the 6 follow-up month , and only 12.5% of patients noted it as “severe” by the 12 follow-up month . In group 1, by the 12 follow-up month, TUI and PUC were detected in 31.1% and 46.7% of patients — while by this time, 44.4% and 15.6% of respondents regarded their condition as “severe” and “very severe”, respectively. However, despite the relatively higher rates of TUI and PUC in groups 1 and 2a at the end of the observation, it is necessary to focus attention on an important fact: as has been mentioned earlier, a certain proportion of patients in each of the groups were completely active immediately after the removal of the urethral catheter. At the same time, during follow-up, insignificant dynamics of rehabilitation of patients were observed at the level of TUC: the increase in the proportion of patients capable of fully controlling urine leakage in groups 1, 2a, and 2b during the entire follow-up period was only 2.2%, 12.0% and 9.6%, respectively.
Data on postoperative LUTS evaluation in patients who achieved TUC turned out to be important: patients from all groups had a significant reduction in obstructive and irritative LUTS from the first follow-up month to the level of 1–2 points, compared to baseline indicators, and their stabilization at the level achieved throughout the follow-up period. It is important that no increase in the level of obstructive LUTS was observed in the modification groups during the observation period, which should be interpreted as the absence of postoperative IVO. This was most significant for patients in group 2b, where the formation of postoperative anastomosis narrowing/stricture was most likely, considering the features of anastomosis. The QoL of patients, as a reflection of the reduction of LUTS, naturally increased in all groups, which was expressed in a decrease in QoL indicators synchronously from the first follow-up month. It is predicted that at the end of the follow-up period, patients who reached the rehabilitation of continence later regarded their condition somewhat worse, compared with those respondents who fully restored the retention function at an earlier time of observation and did not note negative dynamics. It should be noted that the intergroup analysis did not reveal statistically significant differences in IPSS-QoL indicators.
In the complex, the initial TUC was determined primarily by the precision preservation of the elements of the external and internal urethral sphincter and the technical features of the formation of VUA in groups of modified techniques. Evaluation of the outcomes of similar studies also shows that maximum preservation of the axial element of the external sphincter of the MU and the muscle structures included in the internal sphincter of the BN will significantly increase the frequency of complete postoperative continence already in the early follow-up stages and reduce the duration of rehabilitation in the case of incontinence formation of varying grades.
There is enough literature data on the direct relationship between postoperative continence and the length of preserved MU. It is known that the length of the residual stump ≥13 mm is associated with a higher frequency of postoperative continence [27][28]. Along with these data, a systematic review by Mungovan et al. (2017) showed that each preserved 1 mm of MU increased the chance of rehabilitation to TUI by 5–15%, and every 10 mm – by 63–205% [29].
The obtained data mediated the movement of further diagnostic search toward the development of techniques for 'lengthening' the urethral stump with careful control of the positive surgical edge. Thus, van Randenborgh et al. (2004) demonstrated the results of using the precision intraprostatic dissection technique, which made it possible to achieve an average lengthening of the urethra by 1 cm: in the groups of standard (n = 610) vs modified (n = 403) techniques at the terms of the 1 and 6 follow-up months after surgery, TUI was noted in 15.0% and 76.0% of cases vs 33.0% and 89.0% observations, respectively [30]. The evolution of this approach has led to the creation of the intraprostatic urethra preservation technique (EPUP technique), based on the 'telescoping' of the urethra, i.e., release from the prostate for the maximum possible length from the apex to the base of the prostate. In fact, in group 2b, the authors of this paper also used a similar technique in their own modification [22][29].
An equally important point in surgery is the BN-sparing, which can be supplemented with various reconstructive techniques that change its configuration. Hashimoto et al. (2018) presented the results of testing new lateral access to the BN with precision dissection of detrusor fibers at the site of the transition of the BN to the base of the prostate, which allowed achieving high rates of complete continence early during the follow-up: by the postoperative weeks 1 and 4, TUC was determined in 80.0% and 92.0% of patients, respectively [31]. Previously, Tolkach et al. (2015) presented a paper demonstrating a significant advantage related to early continence restoration while preserving and reconstructing BN using the 'deep dorsal stitch' (n = 39) technique related to the standard 'tennis racquet' (n = 45) after 1 month (43.6% vs 26.7% of cases, respectively) and 3 months (60.0% vs 37.8% of cases, respectively) after RP [32].
Study limitations. They are primarily related to differences in the volume of patient samples in the groups, but are corrected using additional modules (corrections for nonequivalence) of statistical methods. In addition, within the framework of this work, no evaluation of the effect of the preserved length of the MU stamp and the length of the intraprostatic urethra on the rehabilitation TUC and PUC was carried out; along with this, the importance of the pelvic floor reconstruction technique and postoperative exercises to strengthen the pelvic diaphragm for the speed to reach the level of TUC or PUC has not been determined. It should be noted that these limitations are not primary related to data analysis in the framework of this study and will be presented in the future.
Conclusion
Concluding the analysis of the functional status of the lower urinary tract of patients who have undergone orRP, it is necessary to highlight the keypoints:
- The use of a modified technique to restore the vesicourethral segment compared to the standard one allows statistically significant reduction of the frequency of UI immediately after the removal of the urethral catheter.
- BN-sparing and 'end-to-end' anastomosis as techniques to restore the vesicourethral segment significantly determine earlier periods of rehabilitation of UC function.
- BN-sparing is a significant technical technique that determines the beginning of improvement in the function of UC from the 1 follow-up months when stability of this indicator is achieved by 4 follow-up months. The frequency of TUI by the 12 follow-up month did not exceed 8.0% vs 31.1% of cases with standard surgical techniques.
- The author's technique for restoring the vesicourethral segment is the most effective in achieving the level of TUC and PUC. The recovery of continence function is noted in patients by the 1 month follow-up, but the achievement of complete stability in this indicator was noted by the 2 follow-up month. TUI in these patients was not detected by the 12 follow-up month.
- The analysis of patients' subjective assessment of the severity of their condition showed that over time, a downward trend in this indicator was observed in all groups; however, in the studied groups, the subjective reduction in the grade of UI was assessed significantly higher than in the control group.
The data obtained by the authors of this study allow considering it necessary and justified to perform more precise and technically complex surgical techniques when performing orRP, aimed at maximizing the sparing of anatomical elements of the sphincter complex, using them in the subsequent restoration of the vesicourethral segment.
References
1. Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479-505. https://doi.org/10.6004/jnccn.2019.0023
2. Seisen T, Vetterlein MW, Karabon P, Jindal T, Sood A, Nocera L, Nguyen PL, Choueiri TK, Trinh QD, Menon M, Abdollah F. Efficacy of local treatment in prostate cancer patients with clinically pelvic lymph node-positive disease at initial diagnosis. Eur Urol. 2018;73(3):452-461. https://doi.org/doi:10.1016/j.eururo.2017.08.01
3. Kogan M.I., Pushkar D.Y., eds. Prostate cancer: from proteomics and genomics to surgery. Moscow: Publishing house “ABV-press”; 2019. ISBN 978-5-903018-64-2. (In Russ.)
4. Bill-Axelson A, Holmberg L, Garmo H, Taari K, Busch C, Nordling S, Häggman M, Andersson SO, Andrén O, Steineck G, Adami HO, Johansson JE. Radical prostatectomy or watchful waiting in prostate cancer — 29-year follow-up. N Engl J Med. 2018;379(24):2319-2329. https://doi.org/10.1056/NEJMoa1807801
5. Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, Aronson WJ, Brawer MK. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med. 2017;377(2):132-142. https://doi.org/10.1056/NEJMoa1615869
6. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, Oxley J, Robinson M, Staffurth J, Walsh E, Bollina P, Catto J, Doble A, Doherty A, Gillatt D, Kockelbergh R, Kynaston H, Paul A, Powell P, Prescott S, Rosario DJ, Rowe E, Neal DE; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. https://doi.org/10.1056/NEJMoa1606220
7. Vernooij RW, Lancee M, Cleves A, Dahm P, Bangma CH, Aben KK. Radical prostatectomy versus deferred treatment for localised prostate cancer. Cochrane Database Syst Rev. 2020;6(6):CD006590. https://doi.org/10.1002/14651858.CD006590.pub3
8. Albertsen PC. Observational studies and the natural history of screen-detected prostate cancer. Curr Opin Urol. 2015;25(3):232-7. https://doi.org/10.1097/MOU.0000000000000157
9. Borregales LD, Berg WT, Tal O, Wambi C, Kaufman S, Gaya JM, Urzúa C, Badani KK. 'Trifecta' after radical prostatectomy: is there a standard definition? BJU Int. 2013;112(1):60-7. https://doi.org/10.1111/bju.12002
10. Xylinas E, Durand X, Ploussard G, Campeggi A, Allory Y, Vordos D, Hoznek A, Abbou CC, de la Taille A, Salomon L. Evaluation of combined oncologic and functional outcomes after robotic-assisted laparoscopic extraperitoneal radical prostatectomy: trifecta rate of achieving continence, potency and cancer control. Urol Oncol. 2013;31(1):99-103. https://doi.org/10.1016/j.urolonc.2010.10.012
11. Johansson E, Steineck G, Holmberg L, Johansson JE, Nyberg T, Ruutu M, Bill-Axelson A; SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891-9. https://doi.org/10.1016/S1470-2045(11)70162-0
12. Salonia A, Abdollah F, Gallina A, Pellucchi F, Castillejos Molina RA, Maccagnano C, Rocchini L, Zanni G, Rigatti P, Montorsi F. Does educational status affect a patient's behavior toward erectile dysfunction? J Sex Med. 2008;5(8):1941-8. https://doi.org/10.1111/j.1743-6109.2008.00810.x
13. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54-61. https://doi.org/10.1016/s0022-5347(17)34871-1
14. Corona G, Lee DM, Forti G, O'Connor DB, Maggi M, O'Neill TW, Pendleton N, Bartfai G, Boonen S, Casanueva FF, Finn JD, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Punab M, Silman AJ, Vanderschueren D, Wu FC; EMAS Study Group. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. 2010;7(4 Pt 1):1362-80. https://doi.org/10.1111/j.1743-6109.2009.01601.x
15. Averbeck MA, Woodhouse C, Comiter C, Bruschini H, Hanus T, Herschorn S, Goldman HB. Surgical treatment of post-prostatectomy stress urinary incontinence in adult men: Report from the 6th International Consultation on Incontinence. Neurourol Urodyn. 2019;38(1):398-406. https://doi.org/10.1002/nau.23845
16. Borges RC, Tobias-Machado M, Gabriotti EN, Dos Santos Figueiredo FW, Bezerra CA, Glina S. Post-radical prostatectomy urinary incontinence: is there any discrepancy between medical reports and patients' perceptions? BMC Urol. 2019;19(1):32. https://doi.org/10.1186/s12894-019-0464-6
17. Tang K, Jiang K, Chen H, Chen Z, Xu H, Ye Z. Robotic vs. Retropubic radical prostatectomy in prostate cancer: a systematic review and an meta-analysis update. Oncotarget. 2017;8(19):32237-32257. https://doi.org/10.18632/oncotarget.13332
18. Hoyland K, Vasdev N, Abrof A, Boustead G. Post-radical prostatectomy incontinence: etiology and prevention. Rev Urol. 2014;16(4):181-8 PMID: 25548545 PMCID: PMC4274175
19. Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. J Urol. 1998;160(4):1301-6. PMID: 9751340
20. Koraitim MM. The male urethral sphincter complex revisited: an anatomical concept and its physiological correlate. J Urol. 2008;179(5):1683-9. https://doi.org/10.1016/j.juro.2008.01.010
21. Cui J, Guo H, Li Y, Chen S, Zhu Y, Wang S, Wang Y, Liu X, Wang W, Han J, Chen P, Nie S, Yin G, Shi B. Pelvic floor reconstruction after radical prostatectomy: a systematic review and meta-analysis of different surgical techniques. Sci Rep. 2017;7(1):2737. https://doi.org/10.1038/s41598-017-02991-8
22. Nunez Bragayrac LA, Hussein AA, Attwood K, Pop E, James G, Osei J, Murekeysoni C, Kauffman EC. Feasibility and continence outcomes of extended prostatic urethral preservation during robot-assisted radical prostatectomy. Prostate Cancer Prostatic Dis. 2020;23(2):286-294. https://doi.org/10.1038/s41391-019-0173-y
23. Ikarashi D, Kato Y, Kanehira M, Takata R, Ito A, Onoda M, Kato R, Matsuura T, Iwasaki K, Obara W. Appropriate preoperative membranous urethral length predicts recovery of urinary continence after robot-assisted laparoscopic prostatectomy. World J Surg Oncol. 2018;16(1):224. https://doi.org/10.1186/s12957-018-1523-2
24. Perlin D.V., Zipunnikov V.P., Dymkov I.N., Shmanev A.O. Functional results of endoscopic extraperitoneal radical intrafascial prostatectomy. Vestn. Urol. 2018;6(1):18-26. (In Russ.) https://doi.org/10.21886/2308-6424-2018-6-1-18-26
25. Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418-24. https://doi.org/10.1097/00005392-199812020-00010
26. Belousov I.I., Tokhtamishyan S.K., Kogan M.I., Chibichyan M.B., Mitusov V.V., Khasigov A.V., Ismailov R.S., invertors; Belousov I.I., Tokhtamishyan S.K., assignee. [Method of forming urethro-urethroanastomosis when performing retropubic radical prostatectomy in patients with prostate cancer]. Russian Federation patent RU 2731790 C1, IPC A61B 17/00. 2020 Sep 08. (In Russ.) EDN: SHGXZB
27. Ando S, Kamei J, Yamazaki M, Sugihara T, Kameda T, Fujisaki A, Kurokawa S, Takayama T, Fujimura T. Longer preserved urethral length in robot-assisted radical prostatectomy significantly contributes to post-operative urinary continence recovery. BJUI Compass. 2021;3(2):184-190. https://doi.org/10.1002/bco2.128
28. Tienza A, Robles JE, Hevia M, Algarra R, Diez-Caballero F, Pascual JI. Prevalence analysis of urinary incontinence after radical prostatectomy and influential preoperative factors in a single institution. Aging Male. 2018;21(1):24-30. https://doi.org/10.1080/13685538.2017.1369944
29. Mungovan SF, Sandhu JS, Akin O, Smart NA, Graham PL, Patel MI. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: a systematic review and meta-analysis. Eur Urol. 2017;71(3):368-378. https://doi.org/10.1016/j.eururo.2016.06.023
30. van Randenborgh H, Paul R, Kübler H, Breul J, Hartung R. Improved urinary continence after radical retropubic prostatectomy with preparation of a long, partially intraprostatic portion of the membraneous urethra: an analysis of 1013 consecutive cases. Prostate Cancer Prostatic Dis. 2004;7(3):253-7. https://doi.org/10.1038/sj.pcan.4500726
31. Hashimoto T, Yoshioka K, Gondo T, Hasama K, Hirasawa Y, Nakashima J, Tachibana M, Ohno Y. The impact of lateral bladder neck preservation on urinary continence recovery after robot-assisted radical prostatectomy. J Endourol. 2018;32(1):40-45. https://doi.org/10.1089/end.2017.0459
32. Tolkach Y, Godin K, Petrov S, Schelin S, Imkamp F. A new technique of bladder neck reconstruction during radical prostatectomy in patients with prostate cancer. Int Braz J Urol. 2015;41(3):455-65. https://doi.org/10.1590/S1677-5538.IBJU.2014.0341
About the Authors
M. I. KoganRussian Federation
Mikhail I. Kogan — M.D., Dr.Sc.(Med), Full Prof., Honored Scientist of the Russian Federation, Head, Dept. of Urology and Human Reproductive Health (with Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
I. I. Belousov
Russian Federation
lgor I. Belousov — M.D., Dr.Sc.(Med), Assoc.Prof.(Docent), Prof., Dept. of Urology and Human Reproductive Health (with Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
V. V. Mitusov
Russian Federation
Valery V. Mitusov — M.D., Dr.Sc.(Med), Assoc.Prof.(Docent), Prof., Dept. of Urology and Human Reproductive Health (with Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
S. K. Tokhtamishyan
Russian Federation
Suren K. Tokhtamishyan — M.D., Postgrad. Student, Dept. of Urology and Human Reproductive Health (with Pediatric Urology and Andrology Course), Rostov State Medical University; Urologist, Urology Division, Azov City Central Hospital.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
R. S. Ismailov
Russian Federation
Ruslan S. Ismailov - M.D., Cand.Sc.(Med), Assist.Prof., Dept. of Urology and Human Reproductive Health (with Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
Review
For citations:
Kogan M.I., Belousov I.I., Mitusov V.V., Tokhtamishyan S.K., Ismailov R.S. Influence of vesicourethral segment reconstruction techniques in radical prostatectomy on urinary continence: evaluation of immediate and long-term outcomes. Urology Herald. 2022;10(4):54-69. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-54-69













































