Scroll to:
Ureteroplasty using oral mucosa graft: a literature review. Update in 2022
https://doi.org/10.21886/2308-6424-2022-10-3-84-97
Abstract
Introduction. The review is aimed at analyzing the worldwide experience in the use of the oral mucosa in ureteroplasty due to benign ureteral strictures.
Objective. To study the features of the use of the oral mucosa in ureteral reconstruction based on a review of the worldwide literature.
Materials and methods. The review was conducted using the PubMed, EMBASE, and the Russian Science Citation Index database. In the first stage, 1013 sources were found, of which 38 articles were selected for inclusion in the review. Of these, 13 studies used an open approach, 15 — robotic, 6 — laparoscopic, 3 — laparoscopic and robotic, 1 — open and laparoscopic. A buccal graft was used in 29 studies and a lingual graft was used in 9 studies.
Results. In total, oral mucosal ureteroplasty was performed 308 times in 306 patients: open technique — 64 times, robotic — 145 times, laparoscopic — 99 times. A buccal graft was used in 67.9% (209/308) of the cases, a lingual graft was used in 32.1% (99/308). Postoperative complications were observed in 15.9% (49/308) of the cases: 12.2% after the open technique, 10.4% after the robotic technique and 20.2% after the laparoscopic technique. With a postoperative follow-up period of 1 to 85 months (average 15.3 months), treatment success was achieved in 92.5% (285/308) of the cases: 93.8% for open technique, 88.2% for robotic, 98.0% for laparoscopic.
Conclusion. The use of the oral mucosa for ureteroplasty due to benign ureteral stricture allows high rates of efficiency and safety. The results of ureteroplasty do not depend on the choice of surgical approach, type of graft and graft transplantation technique.
For citations:
Katibov M.I., Bogdanov A.B., Dovlatov Z.A. Ureteroplasty using oral mucosa graft: a literature review. Update in 2022. Urology Herald. 2022;10(3):84-97. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-3-84-97
INTRODUCTION
Ureteral strictures or obliteration can develop because of many causes: trauma, ischemia, radiation, infectious and inflammatory processes, or iatrogenesis (endoscopic and other surgical interventions). The incidence of ureteral strictures varies depending on the etiological factor: 1.0% after ureteroscopy, 2.0% – 3.0% after radiation therapy, and 5.0% – 24.0% after ureteral stone obstruction for more than 2 months [1].
If strictures of the distal ureter are usually corrected by reconstruction using various methods of reimplantation of the ureter into the bladder, then strictures of the middle or proximal parts of the ureter are much more difficult to reconstruct. Modern approaches to the treatment of strictures of the middle and proximal parts of the ureter include both temporary palliative measures and radical methods aimed at a long-term solution to this problem. Temporary measures include stenting or nephrostomy followed by endoscopic surgery (dilation or ureterotomy). Ureteral stenting and nephrostomy require constant monitoring and repeated procedures, they are poorly tolerated by patients (stent irritation, etc.) and significantly affect the quality of life. Endoscopic procedures can cause an aggravation of the fibrous process in the stricture zone and provide only a short-term effect [2, 3]. Long-term reconstruction options, such as interposition of the ileum or urinary excretion by ureterocutaneostomy, are associated with the development of metabolic disorders and other side effects; therefore, they cannot be used in all patients [4]. Kidney autotransplantation is considered in cases of the ineffectiveness of other approaches and the importance of preserving kidney function, and nephrectomy serves as an extreme measure when other options are not suitable [5].
In this sense, an alternative and promising method of treating ureteral strictures is urethroplasty using the oral mucosa graft (mainly the cheek mucosa). In ureteral stricture, the cheek mucosa was first used in an experiment in monkeys in 1984 [6], and the first experience of clinical use of this technique was noted in 1999 [7]. Despite the fairly good performance and safety indicators, over the almost 25-year clinical history of the use of the oral mucosa in ureteral plastic surgery, the worldwide literature has a small number of works in this field [5, 8–12].
Thus, considering the above-mentioned data, the aim of this study was to study the characteristics of oral mucosa use in ureteral reconstruction based on a review of the worldwide literature. The authors of this study conducted the previous two reviews of the literature in 2018 and 2020 [13, 14]. Considering the appearance of new works on this topic over the past two years, it was decided to update the literature review. Additionally, previous reviews have been devoted to buccal ureteroplasty, and this review of the literature includes work using all oral mucosa variants (cheek and tongue mucosa).
LITERARY SEARCH ALGORITHM
The search for literary sources was carried out by means of using PubMed, EMBASE and the Russian Science Citation Index databases. The search in databases was carried out by the following keywords in English and their analogs in Russian: “ureter”, “ureteral stricture”, “ureteroplasty”, “ureteral reconstruction”, “buccal mucosa”, “lingual mucosa”, “oral mucosa”, “graft”, and “tissue transfer”.
The inclusion criteria were as follows: 1) ureteroplasty in adults or children using any variant of the oral mucosa for primary or recurrent ureteral stricture of benign etiology; 2) publication of the work in a peer-reviewed journal (full text or abstract of the article).
Exclusion criteria were as follows: 1) animal research; 2) kidney transplantation (including autotransplantation); 3) etiology of ureteral stricture secondary to malignant neoplasms; 4) ureteroplasty without the use of oral mucosa graft; 5) conference abstracts; 6) patents of invention; 7) duplicate publications; 8) review works; 9) works describing the technique of the operation without presenting the results of surgical treatment; 10) editorial comments, answers, and letters.
Data compilation was carried out on the following points: surgical access (open, robotic, or laparoscopic), the number of patients, the type of the oral mucosa graft and the technique of its fixation to the ureter wall, localization and length of the ureter stricture, registered complications according to the Clavien-Dindo classification, the duration of the postoperative follow-up, and the success of surgical treatment in the form of restoration of normal patency of the ureter.
Scientific articles published in different journals but devoted to the analysis of the same study with identical data or increasing data as new patients are recruited, are considered an original study. At the same time, the latest study with the highest number of patients is included in the table. A few studies have analyzed the results of two different surgical approaches (open and laparoscopic, robotic, and laparoscopic). If complete data on all the studied items were provided for each surgical access, such studies were included in two tables indicating separate data on the corresponding surgical access. If it was impossible to separate all the data for each surgical access considered, these studies were included in one table for the largest number of surgeries from the two accesses used, indicating the frequency of each access.
A search in these databases revealed 1013 articles. The initial verification of the title and annotation led to the exclusion of 933 studies from them according to various criteria. Subsequently, 38 articles on the results of ureteroplasty using the oral mucosa graft, published in the period from 1999 to 2022, were selected from the remaining 80 articles, considering the inclusion and exclusion criteria in the final review. In 13 studies, ureteroplasty was carried out through open access, 15 —using robotic one, 6 — using laparoscopic access; in three studies, laparoscopic and robotic methods were combined, and open and laparoscopic methods were combined in one study only. At the same time, a buccal graft was used in 29 studies, and a lingual graft in 9 (Fig. 1).
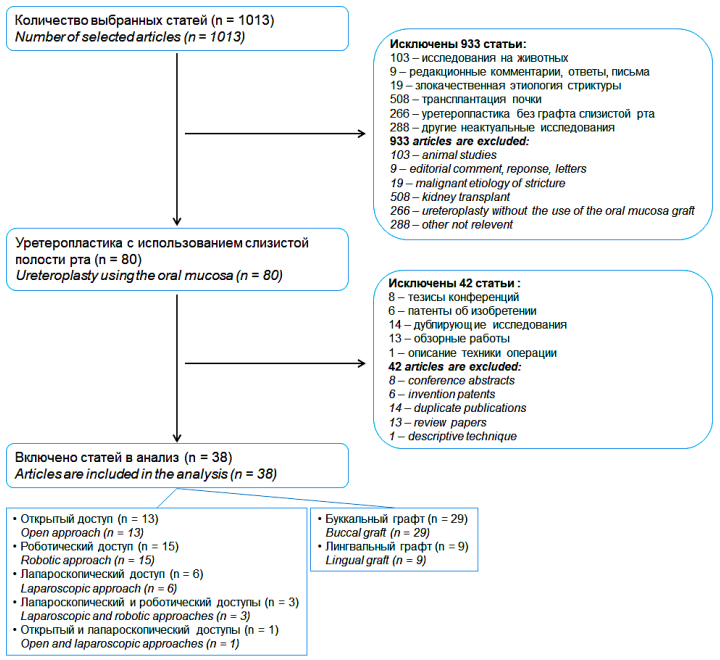
Figure 1. Selection algorithm for non-systematic review
ANALYSIS AND DISCUSSION OF LITERATURE DATA
General clinical and statistical results. A total of 308 ureteroplastic operations were performed using the oral mucosa graft in 306 patients (two patients underwent surgery on each side). Among them, the open technique was used in 64 (20.8%) cases, robotic — in 145 (47.1%), and laparoscopic — in 99 (32.1%).
Age data were indicated in 291 out of 306 patients (95.1%). The average age of patients was 46.6 years with a range of values from 9 to 90 years. At the same time, only one study was of a pediatric nature and included three children aged 9 to 16 years, and all other adult studies included a total of 2 children aged 15 years.
The gender identity of patients was observed in 191 of 306 patients (62.4%). Among them, the proportion of men was 62.3% (119/191), women — 37.7% (72/191).
Information on the extent of ureteral stricture was provided for 300 out of 308 cases of surgical interventions (97.4%). Based on these data, the average value of the stricture length over the entire series of studies was 4.5 cm with a range of indicators from 1 to 15 cm.
Data on ureteral stricture location were given in 305 of 308 surgeries (99.0%). The stricture was most often located in the proximal ureter — in 62.0% (189/305) of cases. In other areas, the stricture occurred with the following frequency: pelvic-ureteral segment — 13.1% (40/305), middle ureter — 10.8% (33/305), distal ureter — 5.6% (17/305), a combination of the pelvic-ureteral segment and proximal ureter — 3.9% (12/305), a combination of the proximal and middle departments of the ureter — 3.0% (9/305), a combination of the middle and distal ureter — 1.0% (3/305), a combination of the proximal and distal ureter — 0.3% (1/305), panureteral stricture — 0.3% (1/305).
The lesion side was observed in 159 out of 308 cases (51.6%), of which 38.4% (61/159) was the right side and 61.6% (98/159) was the left side.
The etiological factor was indicated for 235 of 308 ureteral strictures (76.3%). Most of the causes of stricture development were iatrogenic in nature — 47.7% (112/235). However, it was not possible to accurately detail iatrogenic causes, since in a few studies, iatrogenic etiology was represented by a general term without gradation into all causal categories. Among the most frequent iatrogenic factors, ureteroscopy with contact lithotripsy methods (mainly by means of using laser energy), ureterolithoextraction, open and laparoscopic interventions in the upper urinary tract, and various abdominal and gynecological operations were also observed. Other ethiological variants of ureteral strictures in this series of studies were presented with the following frequency: prolonged presence of a ureteral stone — 21.3% (50/235), congenital stenosis of the pelvic ureteral segment — 15.3% (36/235), tuberculosis — 4.9% (11/235), idiopathic — 4.9% (11/235), radiation therapy — 3.0% (7/235), inflammation — 1.3% (3/235), trauma — 0.9% (2/235), schistosomiasis, endometriosis, and amyloidosis – 0.4% (1/235).
During ureteroplasty, a buccal graft was used in 67.9% (209/308) of cases and a lingual graft was used in 32.1% (99/308). In total, 3 techniques were used to fix the oral mucosa graft to the ureter wall: onlay, augmenting, and tubularization techniques (Fig. 2). The onlay technique was used most frequently in 71.8% (221/308) cases and augmenting and tubularizing techniques were used in 21.8% (67/308) and 6.5% (20/308) cases, accordingly.
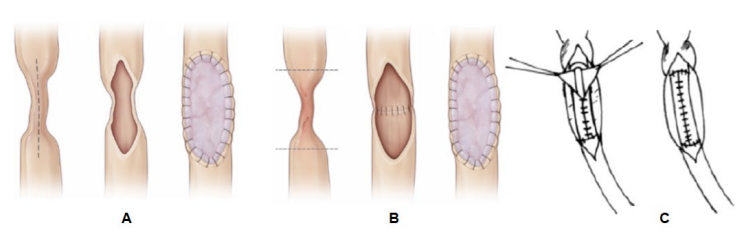
Figure 2. Oral mucosal graft transplantation techniques: A — onlay; B — augmenting; C — tubularizing (A, B — adapted from Z. Lee et al. [15]; C — adapted from A.A. Badawy et al. [16])
Various complications according to the Clavien-Dindo classification occurred in 15.9% (49/308) of cases. At the same time, mild complications (I–II degree) were mainly encountered, and severe complications (≥ III degree) were noted only in 3.6% (11/308) of cases.
The duration of the postoperative follow-up varied from 1 to 85 months, and the average period was 15.3 months. At these follow-up periods, the success rates of treatment in the form of restoration of normal patency of the ureter ranged from 0 to 100%. It should be noted that the extreme indicators of the outcome of treatment in the form of 0 and 100% were mainly since a sufficiently large number of articles (14 studies) were a description of one clinical case. The overall effectiveness of surgical treatment in all studies was 92.5% (285/308). Consequently, the recurrence of the ureteral stricture was recorded in 7.5% (23/308) of observations.
Open ureteroplasty results by means of using the oral mucosa graft. The open ureteroplasty technique using the oral mucosa graft was used a total of 64 times in 62 patients from 14 studies (Table 1). With open access, a buccal graft was used in all the cases. The average length of the ureteral stricture during open surgery was 5.9 cm with an interval of all values from 2.5 to 15.0 cm. The frequency of postoperative complications with the open technique, according to 13 studies in which only the results of open access were presented, was 12.2% (6/49). Of these, only one (2.0%) complication was classified as grade III. The calculation of the complications rate for open surgeries was carried out without considering complications in one of the works in this series, in which, in addition to open access, a laparoscopic approach was used, and the data on complications depending on surgical access were not divided [17]. The duration of postoperative follow-up with an open technique varied from 1 to 85 months and averaged 20.8 months. The success of treatment was achieved in 93.8% (60/64) of cases. The onset of ureteral stricture relapses was observed in the period from 6 to 39 months after surgery.
Table 1. Open ureteroplasty by means of using the oral mucosa graft.
|
Study |
n |
Graft type; Graft transplant technique |
Stricture location |
Average (range) length of stricture, cm |
Complications |
Follow-up, mo. |
Surgical success |
|
Naude, 1999 [7] |
6 |
Buccal; Onlay (n = 5) Tubularized (n = 1) |
UPJ (n = 1) PU (n = 2) MU (n = 4) |
NR |
0 |
3 – 72 |
6/6 (100%) |
|
Shah et al., 2003 [18] |
5 |
Buccal; Onlay |
PU (n = 3) MU (n = 1) Panureteric (n = 1) |
8.6 (5.5 – 15.0) |
0 |
12 |
5/5 (100%)
|
|
Kroepfl et al., 2010 [19] |
6 (7 operations) |
Buccal; Onlay |
PU (n = 2) MU (n = 2) MU + DU (n = 3) |
6.9 (3.0 – 11.0) |
0 |
10 – 85 |
5/7 (71.4%) |
|
Badawy et al., 2010 [16] |
5 |
Buccal; Tubularized |
PU (n = 3) MU (n = 2) |
4.4 (3.5 – 5.0) |
2/5 (40%): Clavien-Dindo I: Fever (n = 1) Clavien-Dindo II: Ileus (n = 1) |
14 – 39 |
5/5 (100%) |
|
Agrawal et al., 2010 [20] |
1 |
Buccal; Onlay |
PU |
7 |
0 |
3 |
1/1 (100%) |
|
Sadhu et al., 2011 [21] |
1 |
Buccal; Onlay |
PU |
8 |
0 |
6 |
1/1 (100%) |
|
Pandey et al, 2014 [22] |
3 |
Buccal; Onlay |
PU |
5.7 (4.0 – 5.7) |
0 |
26 – 50 |
3/3 (100%) |
|
Trapeznikova et al., 2014 [23] |
8 (9 operations) |
Buccal; Onlay |
PU (n = 1) MU (n = 4) DU (n = 4) |
5.1 (3.5 – 7.0) |
0 |
3 – 72 |
8/9 (88.9%) |
|
Tsaturyan et al., 2016 [24] |
5 |
Buccal; Onlay |
PU |
4.2 (2.5 – 5.0) |
3/5 (60%): Clavien-Dindo I: Fever (n = 1) Clavien-Dindo II: Constipation (n = 2) |
26 – 52 |
5/5 (100%) |
|
Sabale et al., 2016 [25] |
1 |
Buccal; Onlay |
PU |
3 |
0 |
8 |
1/1 (100%) |
|
Fahmy et al., 2017 [26] |
1 |
Buccal; Tubularized |
PU |
6 |
0 |
9 |
1/1 (100%) |
|
Hefermehl et al., 2020 [27] |
4 |
Buccal; Onlay |
PU |
4.0 (3.0 – 5.0) |
0 |
12 – 14 |
4/4 (100%) |
|
Date et al., 2021 [28] |
1 |
Buccal; Augmented + ureterocalicostomy |
PU |
8.0 |
1/1 (100%): Clavien-Dindo IIIa: Urinary leak |
12 |
0/1 (0%) |
|
Volkov et al., 2022 [17] |
25 (15 — O, 10 — L)
|
Buccal; Onlay (n = 12) Tubularized (n = 13) |
PU + UPJ (n = 8) PU (n = 5) MU (n = 2) DU (n = 10) |
5.2 (3.0 – 9.0) |
10/25 (40%): Clavien-Dindo I: Urine admixtures in derange discharge (n = 3) Clavien-Dindo II: Pyelonephritis (n = 2) Anemia (n = 1) Pancreatitis exacerbation (n = 1) Pseudomembranous colitis (n = 1) Clavien-Dindo IIIa: Stent migration (n = 1) Clavien-Dindo IIIb: Abdominal wall hernia (n = 1) |
14,7 (1.0 – 57.0) |
24/25 (96.0%) |
| Note. n — number of cases, O — open, L — laparoscopic, UPJ — ureteropelvic junction, PU — proximal ureter, MU — middle ureter, DU — distal ureter, NR — not reported | |||||||
Robotic ureteroplasty results by means of using the oral mucosa graft. Robotic access became the most frequent option when performing ureteroplasty using an oral mucosa graft and was used in 145 cases, of which a buccal graft was used in 124 (85.5%) cases and a lingual graft in 21 (14.5%) (Table 2). The robotic technique was used with an average length of the ureteral stricture of 3.8 cm (range 1.0 – 8.0 cm), which is slightly less than the indicators for open technology. Determination of the frequency of complications after the robotic technique was possible according to 144 observations, among which a complication of any nature occurred in 15 (10.4%) cases, and a complication of ≥ III degree — in 7 (4.9%) cases. These indicators of complications do not differ significantly from the above indicators with an open technique. With robotic interventions, the duration of postoperative follow-up ranged from 1 to 44 months with an average value of 12.9 months. The success of robotic surgery treatment was evaluated in 144 patients, and data from a patient with robotic surgery was included in the overall study results, which included laparoscopic access in addition to robotic access [29]. The success of treatment with the use of robotic technology was 88.2% (127/144). The result obtained is comparable to the success rate of treatment with an open method. Ureteral restenosis after robotic ureteroplasty was diagnosed within a period of 6 weeks to 12 months.
Table 2. Robotic ureteroplasty using the oral mucosa graft
|
Study |
n |
Graft type; Graft transplant technique |
Stricture location |
Average (range) length of stricture, сm |
Complications |
Follow-up, mo. |
Surgical success |
|
|
Zhao et al., 2015 [30] |
4 |
Buccal; Onlay |
UPJ (n = 1) PU (n = 2) PU + DU (n = 1) |
3.9 (1.5 – 6.0) |
0 |
10.7 – 18.6 |
4/4 (100%) |
|
|
Marien et al., 2015 [31] |
2 |
Buccal; Onlay |
PU |
2.3 (1.5 – 3.0) |
0 |
3.5 – 4.0 |
2/2 (100%) |
|
|
Arora et al., 2017 [32] |
1 |
Buccal; Onlay |
PU |
6.0 |
0 |
6 |
1/1 (100%) |
|
|
Ahn et al., 2017 [33] |
3 |
Buccal; Onlay |
UPJ
|
4.3 (2.5 – 6.0) |
1/3 (33.3%): Clavien-Dindo I: Ileus |
13.7 (5.0 – 26.0) |
3/3 (100%) |
|
|
Zampini et al., 2017 [34] |
2 |
Buccal; Onlay |
UPJ |
3.5 – 4.0 |
0 |
17 – 35 weeks |
1/2 (50%) |
|
|
Lee Z et al., 2017 [35] |
12 |
Buccal; Onlay (n = 10) Augmented (n = 2) |
UPJ (n = 4) PU (n = 4) MU (n = 4) |
3.2 (2.0 – 5.0) |
2/12 (16,7%): Clavien-Dindo II: Wound infection, epididymitis (n = 1) Clavien-Dindo IVa: Reintubation (n = 1) |
4 – 30 |
10/12 (83.3%) |
|
|
Zhao et al., 2018 [36] |
19 |
Buccal; Onlay (n = 15) Augmented (n = 4) |
UPJ (n = 5) PU (n = 9) MU (n = 5) |
4.0 (2.0 – 8.0) |
4/19 (21.1%): Clavien-Dindo I-II: Compartment syndrome (n = 1) Wound infection, epididymitis (n = 1) Clavien-Dindo IIIb: Camera-port hernia (n = 1) Clavien-Dindo IVa: Reintubation (n = 1) |
13 – 44 |
17/19 (89.5%) |
|
|
Beysens et al., 2018 [37] |
1 |
Lingual; Onlay |
PU |
2.0 |
0 |
3 |
1/1 (100%) |
|
|
Billah et al., 2020 [38] |
1 |
Buccal; Onlay |
NR |
6.0 |
0 |
NR |
1/1 (100%) |
|
|
Lee M et al., 2020 [39] |
10 |
Buccal; Onlay |
UPJ |
1.0–3.0 |
NR |
20.3 (9.3 – 25.3) |
8/10 (80%) |
|
|
Guliev et al., 2021 [40] |
1 |
Buccal; Onlay |
UPJ |
3.0 |
0 |
4 weeks |
1/1 (100%) |
|
|
Asghar et al., 2021 [41] |
1 |
Buccal; Onlay |
NR |
NR |
0 |
NR |
1/1 (100%) |
|
|
Lee M et al., 2021 [42] |
14 |
Buccal; Onlay (n = 8) Augmented (n = 6) |
PU (n = 12) PU + MU (n = 2) |
5.0 (4.0 – 5.0) |
1/14 (7,1%): Clavien-Dindo IIIa: Compartment syndrome |
24 (14.0 – 39.0) |
13/14 (92.9%) |
|
|
Cheng et al., 2021 [43] |
5 |
Lingual; Onlay Augmented (the frequency of the technique is unknown) |
PU, UPJ + PU (the frequency is unknown) |
4.0 (3.0 – 6.0) |
2/5 (40%) |
8.0 ± 2.1 |
4/5 (80.0%) |
|
|
Lee Z et al., 2021 [15] |
54 |
Buccal; Onlay (n = 43) Augmented (n = 11) |
PU (n = 39) MU (n = 8) PU + MU (n = 7) |
3.0 (1.0 – 8.0) |
3/54 (5,6%): Clavien-Dindo IIIa: Compartment syndrome (n = 1) Clavien-Dindo IIIb: Camera port hernia (n = 1) Clavien-Dindo IVa: Reintubation (n = 1) |
27.5 (21.3 – 38.0) |
47/54 (87.0%) |
|
|
Fan et al., 2021 [44] |
2 |
Lingual; Augmented |
PU |
4.0 – 5.0 |
1/2 (50%): Clavien-Dindo I: Numbness of tongue |
7 |
2/2 (100%) |
|
|
Yang et al., 2022 [45] |
12
|
Lingual; Onlay (n = 6); Augmented (n = 6) |
PU |
4.7 (3.0 – 6.5) |
1/12 (8,3%): Clavien-Dindo II: Urinary tract infection |
15 (13 – 27) |
11/12 (91.7%) |
|
| Note. n — number of cases, UPJ — ureteropelvic junction, PU — proximal ureter, MU — middle ureter, DU — distal ureter, NR — not reported | ||||||||
Laparoscopic ureteroplasty results using the oral mucosa graft. A laparoscopic approach for ureteroplasty using a graft of the oral mucosa was applied in 99 patients. Almost all cases of its use occurred in the last 3 years: 9 out of 10 studies involving the laparoscopic method were published from 2020 to 2022 (Table 3). A distinctive feature of the use of laparoscopic access can be recognized as the prevalence of the use of a lingual graft, which became a plastic material in 78.8% (78/99) of the patients from the laparoscopic series of studies, while a buccal graft was used only in 21.2% (21/99) of the observations. The laparoscopic technique was applied with an average length of ureteral stricture of 3.6 cm (range 2.0 – 9.0 cm), which turned out to be comparable to the robotic access indicators. Since in several studies, the results of laparoscopic access were presented together with another approach used in this study (open or robotic) without separating them by surgical methods, it was possible to determine a few indicators based on the data of only a part, and not all patients who underwent laparoscopic intervention. Various complications after laparoscopic surgery were found in 20.2% (18/89) of cases, which turned out to be significantly higher than similar indicators for open and robotic operations. However, severe complications of grade III with the laparoscopic technique, on the contrary, were noted less frequently than after the other two surgical access options and amounted to 1.1% (1/89) of cases. The duration of postoperative follow-up with the laparoscopic approach ranged from 1 to 80 months with an average of 12.9 months. The success of treatment with laparoscopic access was noted in 98.0% (97/99) of cases. According to this indicator, the laparoscopic method was superior to the open and robotic approaches.
Table 3. Laparoscopic ureteroplasty results using the oral mucosa graft
|
Study |
n |
Graft type; Graft transplant technique |
Stricture location |
Average (range) length of stricture, сm |
Complications |
Follow-up, mo. |
Surgical success |
|
|
Li et al., 2016 [46] |
1 |
Lingual; Onlay |
PU |
3.0 |
0 |
9 |
1/1 (100%) |
|
|
Huang et al., 2020 [47] |
1 |
Lingual; Onlay |
PU |
2.5 |
0 |
9 |
1/1 (100%) |
|
|
Menegola et al., 2020 [48] |
1 |
Buccal; Onlay |
PU |
NR |
0 |
1 |
1/1 (100%) |
|
|
Guliev et al., 2021 [49] |
10 |
Buccal; Onlay |
UPJ (n = 6) PU (n = 4) |
3.6 (2.0 – 9.0) |
1/10 (10%): Clavien-Dindo I: Fever |
10.1 (3.0 – 18.0) |
10/10 (100%) |
|
|
Fan et al., 2021 [44] |
8 |
Lingual; Augmented |
PU |
3.3 (3.0 – 4.0) |
1/8 (12,5%): Clavien-Dindo II: Wound infection, urinary tract infection |
13.0 (7.0 – 20.0) |
8/8 (100%) |
|
|
Cheng et al., 2021 [43] |
11 |
Lingual; Onlay Augmented (the frequency of the technique is unknown) |
PU, UPJ + PU (the frequency is unknown) |
4.0 (3.0 – 6.0) |
3/11 (27.3%) |
15.5 ± 4.5 |
11/11 (100%) |
|
|
Wang et al., 2021 [50] |
16 |
Lingual; Onlay |
PU |
3.9 (3.0 – 4.5) |
7/16 (43,8%): Clavien-Dindo I: Numbness of tongue (n = 4), Oral ulcer (n = 1) Clavien-Dindo II: Urinary tract infection (n = 2) |
17.9 ± 5.2 |
16/16 (100%) |
|
|
Gao et al., 2021 [51] |
1 |
Lingual; (PU) + Ureterovesical reimplantation (DU) |
3 strictures: 1 PU and 2 DU |
3.5 (PU) |
0 |
6.0 |
1/1 (100%) |
|
|
Liang et al., 2022 [29] |
41 (40 — L, 1 — R) |
Lingual; Onlay (n = 24) Augmented (n = 17) |
UPJ (n = 7) PU (n = 32) MU (n = 2) |
4.8 (2.0 – 8.0) |
6/41 (14.6%): Clavien-Dindo I: Fever (n = 4) Lymphorrhagia (n = 1) Clavien-Dindo IIIa: Severe urinary tract infection (nephrostomy) (n = 1) |
35.0 (13.0 – 80.0) |
40/41 (97.6%) |
|
| Note. n — number of cases, L — laparoscopic, R — robotic, UPJ — ureteropelvic junction, PU — proximal ureter, MU — middle ureter, DU — distal ureter, NR — not reported |
|
|||||||
Thus, the effectiveness of ureteroplasty using the oral mucosal graft turned out to be sufficiently high and close with all the surgical approaches used. The spread of success rates in treatment within 10.0% (88.2 – 98.0%) should be considered acceptable. In addition, comparable treatment results were achieved regardless of other factors: the type of the oral mucosal graft (buccal or lingual), oral mucosal graft techniques (onlay, augmenting, or tubularizing techniques), wrapping the graft with fatty tissue / omentum or without performing this technique, and the nature of stricture (primary or recurrent). For example, the success of treatment with a buccal graft was 90.4% (189/209), lingual graft — 97.0% (96/99). The difference in favor of the lingual graft can be explained by a shorter postoperative follow-up and the number of patients during surgeries using this type of graft.
CONCLUSION
The use of the oral mucosa graft during ureteral reconstruction due to its stricture of benign etiology allows high restoration rates of the ureter. While using this technique, the risk of postoperative complications, especially severe ones, is quite low. The results of urethroplasty using the oral mucosa graft do not depend significantly on the choice of surgical access, the type of graft, and the graft transplant technique. Considering the presented data, ureteroplasty by means of using the oral mucosa graft can be considered as an alternative method of treating extended ureteral stricture and included in the category of recommended approaches for a further study of the management of patients with this disease at specialized centers of reconstructive plastic urology.
References
1. Lucas JW, Ghiraldi E, Ellis J, Friedlander JI. Endoscopic Management of Ureteral Strictures: an Update. Curr Urol Rep. 2018;19(4):24. https://doi.org/10.1007/s11934-018-0773-4
2. Reus C, Brehmer M. Minimally invasive management of ureteral strictures: a 5-year retrospective study. World J Urol. 2019;37(8):1733-1738. https://doi.org/10.1007/s00345-018-2539-5
3. Tyritzis SI, Wiklund NP. Ureteral strictures revisited…trying to see the light at the end of the tunnel: a comprehensive review. J Endourol. 2015;29(2):124-36. https://doi.org/10.1089/end.2014.0522
4. Roth JD, Koch MO. Metabolic and Nutritional Consequences of Urinary Diversion Using Intestinal Segments to Reconstruct the Urinary Tract. Urol Clin North Am. 2018;45(1):19-24. https://doi.org/10.1016/j.ucl.2017.09.007
5. Heijkoop B, Kahokehr AA. Buccal mucosal ureteroplasty for the management of ureteric strictures: A systematic review of the literature. Int J Urol. 2021;28(2):189-195. https://doi.org/10.1111/iju.14426
6. Somerville JJ, Naude JH. Segmental ureteric replacement: an animal study using a free non-pedicled graft. Urol Res. 1984;12(2):115-9. https://doi.org/10.1007/BF00257176
7. Naude JH. Buccal mucosal grafts in the treatment of ureteric lesions. BJU Int. 1999;83(7):751-4. https://doi.org/10.1046/j.1464-410x.1999.00019.x
8. Del Pozo Jiménez G, Castillón-Vela I, Carballido Rodríguez J. Uso de injerto de mucosa oral en el tratamiento de estenosis ureterales extensas: revisión de conjunto [Buccal mucosa graft for the treatment of long ureteral stenosis: Bibliographic review.]. Arch Esp Urol. 2017;70(4):445-453. (In Spanish) PMID: 28530624
9. Waldorf B, Lee Z, Kidd L, Kaplan J, Harris A, Metro M, Liu J, Eun D. Robotic Buccal Ureteroplasty: a Review of the Current Literature. Curr Urol Rep. 2017;18(5):40. https://doi.org/10.1007/s11934-017-0683-x
10. Lee Z, Keehn AY, Sterling ME, Metro MJ, Eun DD. A Review of Buccal Mucosa Graft Ureteroplasty. Curr Urol Rep. 2018;19(4):23. https://doi.org/10.1007/s11934-018-0772-5
11. Yang K, Fan S, Li Z, Guan H, Zhang P, Li X, Zhou L. Lingual mucosa graft ureteroplasty for ureteral stricture: a narrative review of the current literature. Ann Palliat Med. 2021;10(4):4840-4845. https://doi.org/10.21037/apm-20-2339
12. Guliev BG, Komyakov BK, Avazkhanov ZhP. Buccal grafting of extended strictures of proximal ureter (Review). Eksperimentalnaya i klinicheskaya urologiya. 2019;(4):86-91. (In Russ.) https://doi.org/10.29188/2222-8543-2019-11-4-86-91
13. Katibov MI, Polyakov NV, Keshishev NG, Apolikhin OI, Kaprin AD. Use of buccal graft for the management of ureteral strictures. Urologiia. 2018;(1):138-142. (In Russ.) https://doi.org/10.18565/urology.2018.1.138-142
14. Katibov MI, Bogdanov AB, Dovlatov ZA. Buccal urethroplasty: 2020 literature review update. Eksperimentalnaya i klinicheskaya urologiya. 2020;(3):118-123. (In Russ.) https://doi.org/10.29188/2222-8543-2020-12-3-118-123
15. Lee Z, Lee M, Koster H, Lee R, Cheng N, Jun M, Slawin J, Zhao LC, Stifelman MD, Eun DD. A Multi-Institutional Experience With Robotic Ureteroplasty With Buccal Mucosa Graft: An Updated Analysis of Intermediate-Term Outcomes. Urology. 2021;147:306-310. https://doi.org/10.1016/j.urology.2020.08.003
16. Badawy AA, Abolyosr A, Saleem MD, Abuzeid AM. Buccal mucosa graft for ureteral stricture substitution: initial experience. Urology. 2010;76(4):971-975. https://doi.org/10.1016/j.urology.2010.03.095
17. Volkov A.A, Budnik NV, Zuban O, Mustapaev ID, Abdulaev MA, Muziev AV. Buccal ureteroplasty options, techniques, long-term results. Issledovaniâ i praktika v medicine. 2022;9(2):86-95. (In Russ.) https://doi.org/10.17709/2410-1893-2022-9-2-7
18. Shah SA, Ranka P, Visnagara M, Dodia S, Jain R. Use of buccal mucosa as onlay graft technique for benign ureteric strictures. Indian J Urol. 2003;20(1):28-32. URL: https://www.indianjurol.com/text.asp?2003/20/1/28/37120
19. Kroepfl D, Loewen H, Klevecka V, Musch M. Treatment of long ureteric strictures with buccal mucosal grafts. BJU Int. 2010;105(10):1452-1455. https://doi.org/10.1111/j.1464-410X.2009.08994.x
20. Agrawal V, Dassi V, Andankar MG. Buccal mucosal graft onlay repair for a ureteric ischemic injury following a pyeloplasty. Indian J Urol. 2010;26(1):120-122. https://doi.org/10.4103/0970-1591.60458
21. Sadhu S, Pandit K, Roy MK, Bajoria SK. Buccal mucosa ureteroplasty for the treatment of complex ureteric injury. Indian J Surg. 2011;73(1):71-72. https://doi.org/10.1007/s12262-010-0199-9
22. Pandey A, Dican R, Beier J, Keller H. Buccal mucosal graft in reconstructive urology: uses beyond urethral stricture. Int J Urol. 2014;21(7):732-734. https://doi.org/10.1111/iju.12403
23. Trapeznikova MF, Bazaev VV, Shibaev AN, Luk'ianchikov AG, Vinogradov AV. Replacement plastic reconstruction of extended ureteral stricture using buccal mucosa autograft. Urologiia. 2014;(2):16-19. (In Russ.) eLIBRARY ID: 21710626 EDN: SHCLCT
24. Tsaturyan A, Akopyan K, Levonyan A, Tsaturyan A. Long ureteric stricture replacement by buccal mucosa graft: an Armenian experience case series report. Cent European J Urol. 2016;69(2):217-220. https://doi.org/10.5173/ceju.2016.757
25. Sabale VP, Thakur N, Kankalia SK, Satav VP. A case report on buccal mucosa graft for upper ureteral stricture repair. Urol Ann. 2016;8(4):474-477. https://doi.org/10.4103/0974-7796.192092
26. Fahmy O, Schubert T, Khairul-Asri MG, Stenzl A, Gakis G. Total proximal ureter substitution using buccal mucosa. Int J Urol. 2017;24(4):320-323. https://doi.org/10.1111/iju.13307
27. Hefermehl LJ, Tritschler S, Kretschmer A, Beck V, Stief CG, Schlenker B, Strittmatter F. Open ureteroplasty with buccal mucosa graft for long proximal strictures: A good option for a rare problem. Investig Clin Urol. 2020;61(3):316-322. https://doi.org/10.4111/icu.2020.61.3.316
28. Date JA, Nathani AS, Shivde SR, Kulkarni CR. Combined ureterocalicostomy with buccal mucosa graft ureteroplasty in complex upper ureteral stricture: A rare case of reconstruction. Urol Ann. 2021;13(2):186-189. https://doi.org/10.4103/UA.UA_80_20
29. Liang C, Wang J, Hai B, Xu Y, Zeng J, Chai S, Chen J, Zhang H, Gao X, Cheng G, Yang X, Hou T, Li W, Xiao X, Li B. Lingual Mucosal Graft Ureteroplasty for Long Proximal Ureteral Stricture: 6 Years of Experience with 41 Cases. Eur Urol. 2022:S0302-2838(22)02340-5. https://doi.org/10.1016/j.eururo.2022.05.006
30. Zhao LC, Yamaguchi Y, Bryk DJ, Adelstein SA, Stifelman MD. Robot-Assisted Ureteral Reconstruction Using Buccal Mucosa. Urology. 2015;86(3):634-638. https://doi.org/10.1016/j.urology.2015.06.006
31. Marien T, Bjurlin MA, Wynia B, Bilbily M, Rao G, Zhao LC, Shah O, Stifelman MD. Outcomes of robotic-assisted laparoscopic upper urinary tract reconstruction: 250 consecutive patients. BJU Int. 2015;116(4):604-611. https://doi.org/10.1111/bju.13086
32. Arora S, Campbell L, Tourojman M, Pucheril D, Jones LR, Rogers C. Robotic Buccal Mucosal Graft Ureteroplasty for Complex Ureteral Stricture. Urology. 2017;110:257-258. https://doi.org/10.1016/j.urology.2017.06.037
33. Ahn JJ, Shapiro ME, Ellison JS, Lendvay TS. Pediatric Robot-assisted Redo Pyeloplasty With Buccal Mucosa Graft: A Novel Technique. Urology. 2017;101:56-59. https://doi.org/10.1016/j.urology.2016.12.036
34. Zampini AM, Nelson R, Zhang JJH, Reese J, Angermeier KW, Haber GP. Robotic Salvage Pyeloplasty With Buccal Mucosal Onlay Graft: Video Demonstration of Technique and Outcomes. Urology. 2017;110:253-256. https://doi.org/10.1016/j.urology.2017.07.023
35. Lee Z, Waldorf BT, Cho EY, Liu JC, Metro MJ, Eun DD. Robotic Ureteroplasty with Buccal Mucosa Graft for the Management of Complex Ureteral Strictures. J Urol. 2017;198(6):1430-1435. https://doi.org/10.1016/j.juro.2017.06.097
36. Zhao LC, Weinberg AC, Lee Z, Ferretti MJ, Koo HP, Metro MJ, Eun DD, Stifelman MD. Robotic Ureteral Reconstruction Using Buccal Mucosa Grafts: A Multi-institutional Experience. Eur Urol. 2018;73(3):419-426. https://doi.org/10.1016/j.eururo.2017.11.015
37. Beysens M, Groote R, Van Haute C, Tailly T, Lumen N, Decaestecker K. Robotic lingual mucosal onlay graft ureteroplasty for proximal ureteral stricture. Eur Urol Suppl. 2018; 17(2):e1935.
38. Billah MS, Stifelman M, Munver R, Tsui J, Lovallo G, Ahmed M. Single port robotic assisted reconstructive urologic surgery-with the da Vinci SP surgical system. Transl Androl Urol. 2020;9(2):870-878. https://doi.org/10.21037/tau.2020.01.06
39. Lee M, Lee Z, Strauss D, Jun MS, Koster H, Asghar AM, Lee R, Chao B, Cheng N, Ahmed M, Lovallo G, Munver R, Zhao LC, Stifelman MD, Eun DD. Multi-institutional Experience Comparing Outcomes of Adult Patients Undergoing Secondary Versus Primary Robotic Pyeloplasty. Urology. 2020;145:275-280. https://doi.org/10.1016/j.urology.2020.07.008
40. Guliev BG, Ilyin DM, Avazkhanov ZhP. Robot-assisted pyeloplasty with buccal mucosa graft for the management of an extended recurrent ureteropelvic junction stricture. Vestn. Urol. 2021;9(4):122–126. (In Russ.) https://doi.org/10.21886/2308–6424–2021–9-4–122–126
41. Asghar AM, Lee Z, Lee RA, Slawin J, Cheng N, Koster H, Strauss DM, Lee M, Reddy R, Drain A, Lama-Tamang T, Jun MS, Metro MJ, Ahmed M, Stifelman M, Zhao L, Eun DD. Robotic Ureteral Reconstruction in Patients with Radiation-Induced Ureteral Strictures: Experience from the Collaborative of Reconstructive Robotic Ureteral Surgery. J Endourol. 2021;35(2):144-150. https://doi.org/10.1089/end.2020.0643
42. Lee M, Lee Z, Koster H, Jun M, Asghar AM, Lee R, Strauss D, Patel N, Kim D, Komaravolu S, Drain A, Metro MJ, Zhao L, Stifelman M, Eun DD. Intermediate-term outcomes after robotic ureteral reconstruction for long-segment (≥4 centimeters) strictures in the proximal ureter: A multi-institutional experience. Investig Clin Urol. 2021;62(1):65-71. https://doi.org/10.4111/icu.20200298
43. Cheng S, Fan S, Wang J, Xiong S, Li X, Xu Y, Li Z, Guan H, Zhang P, Zhu H, Huang C, Zhang L, Yang K, Li X, Zhou L. Laparoscopic and robotic ureteroplasty using onlay flap or graft for the management of long proximal or middle ureteral strictures: our experience and strategy. Int Urol Nephrol. 2021;53(3):479-488. https://doi.org/10.1007/s11255-020-02679-5
44. Fan S, Yin L, Yang K, Wang J, Li X, Xiong S, Yu X, Li Z, Guan H, Zhu H, Zhang P, Li X, Zhou L. Posteriorly Augmented Anastomotic Ureteroplasty with Lingual Mucosal Onlay Grafts for Long Proximal Ureteral Strictures: 10 Cases of Experience. J Endourol. 2021;35(2):192-199. https://doi.org/10.1089/end.2020.0686
45. Yang K, Fan S, Wang J, Yin L, Li Z, Xiong S, Han G, Meng C, Zhang P, Li X, Zhou L. Robotic-assisted Lingual Mucosal Graft Ureteroplasty for the Repair of Complex Ureteral Strictures: Technique Description and the Medium-term Outcome. Eur Urol. 2022;81(5):533-540. https://doi.org/10.1016/j.eururo.2022.01.007
46. Li B, Xu Y, Hai B, Liu B, Xiang Y, Hua X, Hou T. Laparoscopic onlay lingual mucosal graft ureteroplasty for proximal ureteral stricture: initial experience and 9-month follow-up. Int Urol Nephrol. 2016;48(8):1275-1279. https://doi.org/10.1007/s11255-016-1289-9
47. Huang BW, Wang J, Zhang P, Li Z, Bi SC, Wang Q, Yue CB, Yang KL, Li XS, Zhou LQ. [Application of indocyanine green in complex upper urinary tract repair surgery]. Beijing Da Xue Xue Bao Yi Xue Ban. 2020;52(4):651-656. https://doi.org/10.19723/j.issn.1671-167X.2020.04.010
48. Menegola C, Tavares PM, Batezini NS, Gorgen ARH, Rosito TE. Laparoscopic ureteroplasty with buccal mucosa graft for long proximal ureteral stenosis: A step by step video. Int Braz J Urol. 2020;46(6):141-142. https://doi.org/10.1590/S1677-5538.IBJU.2018.0830
49. Guliev BG, Komyakov BK, Avazkhanov JP. Laparoscopic substitution of the proximal ureter using buccal mucosa. Urologiia. 2021;(3):13-19. (In Russ.) https://doi.org/10.18565/urology.2021.3.13–19
50. Wang J, Zhang B, Fan J, Cheng S, Fan S, Yin L, Li Z, Guan H, Yang K, Li X. The application of the "omental wrapping" technique with autologous onlay flap/graft ureteroplasty for the management of long ureteral strictures. Transl Androl Urol. 2021;10(7):2871-2878. https://doi.org/10.21037/tau-21-305
51. Gao X, Liang C, Wang J, Xiao X, Li B. Laparoscopic onlay lingual mucosal graft ureteroplasty combined with ureterovesical reimplantation for one-stage reconstruction of complex ureteral strictures: a case report. Transl Androl Urol. 2021;10(10):3907-3914. https://doi.org/10.21037/tau-21-639
52. Liang C, Wang J, Hai B, Xu Y, Zeng J, Chai S, Chen J, Zhang H, Gao X, Cheng G, Yang X, Hou T, Li W, Xiao X, Li B. Lingual Mucosal Graft Ureteroplasty for Long Proximal Ureteral Stricture: 6 Years of Experience with 41 Cases. Eur Urol. 2022;82(2):193-200. https://doi.org/10.1016/j.eururo.2022.05.006
About the Authors
M. I. KatibovRussian Federation
Magomed I. Katibov — M.D., Dr.Sc.(Med), Assoc.Prof. (Docent), Prof., Dept. of Urology; Head, Urological Division
89 Laptiyeva St., Makhachkala, 367018, Russian Federation
Lenin Sq., Makhachkala, 367012, Russian Federation
A. B. Bogdanov
Russian Federation
Andrey B. Bogdanov — M.D., Сand.Sc.(Med); Assoc.Prof., Dept. of Urology and Surgical Andrology; Urologist, Urological Division
5 2nd Botkin Ave, Moscow, 125284, Russian Federation
2/1 Barrikadnaya St., Moscow, 125993, Russian Federation
Z. A. Dovlatov
Russian Federation
Zyaka A. Dovlatov — M.D., Dr.Sc.(Med); Assoc.Prof., Dept.
of Urology and Surgical Andrology
2/1 Barrikadnaya St., Moscow, 125993, Russian Federation
Review
For citations:
Katibov M.I., Bogdanov A.B., Dovlatov Z.A. Ureteroplasty using oral mucosa graft: a literature review. Update in 2022. Urology Herald. 2022;10(3):84-97. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-3-84-97













































