Scroll to:
Percutaneous nephrolitholapaxy performed under ultrasound and endovisual guidance: evaluation of the factors affecting the immediate outcomes
https://doi.org/10.21886/2308-6424-2022-10-2-05-18
Abstract
Introduction. In recent years, ultrasound and endovisual guidance have been introduced into clinical practice when performing percutaneous nephrolitholapaxy.
Objective. To identify the most significant factors that influence the frequency of complete elimination of stones and the development of complications in percutaneous nephrolitholapaxy performed under ultrasound and endovisual guidance.
Materials and methods. We analyzed the results of the treatment of 515 kidney stone patients who underwent percutaneous nephrolitholapaxy under ultrasound navigation and endovideoscopic guidance using a new method developed by us.
Results. The average duration of the operation was 77.2 ± 1.9 min. Most of the operations were performed through one puncture access (95.1%) and in one stage (91.8%) with complete stone elimination in 80.6% of the cases. The degree of decrease in hemoglobin in the postoperative period was only 12.18 ± 0.6 g/l. The frequency of postoperative complications was 29.3%, in most cases there were 1 - 2 severity grades according to Clavien-Dindo. As a result of multivariate regression analysis, independent predictors influencing the frequency of complete stone eradication were the number of stones (p = 0.012), the fact of the presence of staghorn stone (p = 0.016), the number of stages of surgical intervention (p = 0.001). Correlation analysis revealed a statistically significant negative correlation between body mass index and the occurrence of complications (p = 0.005), a positive correlation between the presence of urinary tract infection and the occurrence of complications (p = 0.048), a positive correlation between the grade of blood loss and the occurrence of expectation (р < 0.001).
Conclusions. Percutaneous nephrolitholapaxy under ultrasound and endovisual guidance without the use of X-rays is an effective intervention for most patients with kidney stones. Complete stone eradication depends on the number of stones, the presence of staghorn stone, and the number of stages of surgery. The incidence of complications is significantly affected by the body mass index, the presence of urinary infection and the degree of decrease in hemoglobin during surgery.
For citations:
Atduev V.A., Abramov D.V., Dyrdik M.B., Danilov A.A., Ledyaev D.S., Gasrataliev V.E., Stroganov A.B. Percutaneous nephrolitholapaxy performed under ultrasound and endovisual guidance: evaluation of the factors affecting the immediate outcomes. Urology Herald. 2022;10(2):5-18. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-2-05-18
Introduction
Percutaneous nephrolitholapaxy (PCNL) is currently a standard method of treatment for kidney stones. This low invasive treatment method for patients with large and staghorn kidney stones showed its effectiveness and a low rate of complications [1-10].
Percutaneous low invasive surgeries on kidneys have specific complications that include inflammation, hemorrhage, pleural and abdominal cavity lesion, and the need for repeated operation [9-19]. PCNL does not always provide complete stone elimination in patients with large and multiple kidney stones, especially staghorn stones. In such cases, surgeons must perform several surgical interventions, which increase the risks of perioperative complications [19-21].
Percutaneous interventions currently require radiograph guidance [7][22-24]. X-ray guidance has certain drawbacks that include radiological exposure of medical personnel and patients and the need for a specialized surgical ward with expensive equipment. Although medical personnel use special protection during such surgeries, percutaneous interventions with radioscopy can often lead to complications in them [24-28]. Long-term radioscopy during percutaneous surgeries can lead to the development of a cataract [27]. The maximum radiation dose to the surgeon’s eyes is observed after 1200 PCNL surgeries within one year, with a total full-body radiation dose of 150 mSv [28]. Besides, medical personnel must wear special protection, the weight of which significantly disrupts surgeon thermoregulation and complicates manipulation during surgery.
PCNL technique is continually evolving with the development of new safer technologies [29]. Lately, ultrasonic scanning methods have been implemented in clinical practice as an alternative to X-ray guidance during PCNL [30-37]. Thus, a complex clinical evaluation of the efficiency of new PCNL technology is relevant.
The study aimed to identify the most significant factors that affect the rate of complete elimination of kidney stones and the development of complications in patients after PCNL performed under ultrasound (US) and endovisual guidance.
Materials and Methods
A total of 515 patients (81.0%) patients who underwent PCNL under an original method of US and endovisual guidance (Patent for Invention of the Russian Federation No. 2755226 dated March 15, 2021) were studied [38]. The patients were operated in the urological clinic of the Privolzhskiy Regional Medical Center of the Nizhny Novgorod Russian Federal Clinical Research Center. The analysis and stratification of complications after PCNL were made using the modified classification of surgical complications of Clavien-Dindo [39-44].
Percutaneous nephrolitholapaxy under ultrasound and endovisual guidance. Specialists from the urological clinic of the Privolzhskiy Regional Medical Center in 2007 developed and advanced an original method of PCNL performed under US and endovisual control [38]. By now, over 4,000 PCNL surgeries were performed using this method. There are several technical peculiarities in this type of surgery. The patient is positioned on the “healthy” side in the dorsal-lateral position so that the kidney to be operated on would be on top. The costovertebral angle is increased by lowering the head and leg ends of the surgical table relative to the axis at the level of L4-L5 (Fig. 1).

Figure 1. Dorsal-lateral positioning of the patient when preparing for percutaneous nephrolitholapaxy under ultrasound and endovisual control
An ultrasonic scanner Phillips HD7 (Koninklijke Philips N.V., Philips Medical Systems Nederland B.V., Heerlen, The Netherlands) is set in the puncture mode. US investigation of the kidney and surrounding organs is performed, and US landmarks are differentiated (kidney shape and dimensions, type of the pyelocalyceal system (PCS), the number, configuration, and dimensions of kidneys). Further, the optimal direction for puncture is identified. The puncture channel should not cross neighboring organs (liver, spleen, lung sinus, intestine, peritoneal reflection, and large vessels). The lack of PCS dilation does not prevent its puncture. In this case, the final puncture point will be at the outer edge of the stone in the pelvis or calix. The needle is inserted toward the chosen point, considering the puncture landmarks on the screen. The needle is gradually inserted according to the respiratory movements of the patient, i.e. during the pauses at the height of inhalation or expiration. During the manipulation, the puncture needle end should be visible as a bright light point. When the needle penetrates the cavity, the internal part of the needle is extracted. A syringe is connected to the needle to create a vacuum effect and get urine. Further, a super-stiff guidewire is introduced, which must be controlled by US visualization. When the guidewire end is moved over the needle end, it rolls in a coil because of its construction and prevents damage to distal tissues. The guidewire is left inside, and the needle is removed from the guidewire and extracted.
The next stage includes dilation of the puncture channel to 12 Ch using a plastic dilator, which is placed on the guidewire and moved to the guidewire coil (natural obstacle) by rotational movements. Further, the dilator should be extracted and a 10 Ch ureteroscope should be placed on the guidewire (Karl Storz SE GmbH & Co. KG., Tuttlingen, Germany). The ureteroscope is inserted in the nephrostomy channel over the guidewire under irrigation. The punctured tissues are visualized. Surgeons can see and diagnose damage to the intestine and pleural cavity and other accidental injuries before installing the Amplatz tube (Boston Scientific Corp., San Jose, CA, USA) and before the beginning of the main stage of surgery. If there are any technical obstacles that prevent surgical intervention, the prepared channel should be left.
When the puncture is successful, the ureteroscope passes through the layers of the abdominal wall, the retroperitoneum, and the kidney parenchyma in the kidney cavity. Further, nephroscopy is performed (Karl Storz SE GmbH & Co. KG., Tuttlingen, Germany). A safety guidewire is introduced in the ureteropelvic junction and ureter (Fig. 2). A safety guidewire remains introduced and the ureteroscope should be removed. After that, the ureteroscope is introduced again in the puncture channel along the safety wire and the channel is examined again. When the tool is inserted into the renal cavitary system, all cavities should be re-examined. The stone and the possibility of its removal should be evaluated. Under visualization, an optimal place is chosen in the renal system for further channel dilation and the introduction of an Amplatz tube. A super-stiff guidewire is inserted again in this place; its coil is placed in the PCS area. The ureteroscope is removed from the guidewire. The channel is dilated to 26 Ch using Alken dilators (Karl Storz SE GmbH & Co. KG., Tuttlingen, Germany).

Figure 2. Manipulations with a ureteroscope along the guidewire installed in the ureteropelvic junction and re-control of the stone
For safe channel dilation with simultaneous insertion to the final point, it is necessary to fix the external nephroscope tube equipped with an Amplatz tube, which prevents their disposition when moving into the kidney and accidental injury by the external nephroscope tube. The authors used an original advanced construction of the fixing element (Karl Storz SE GmbH & Co. KG., Tuttlingen, Germany), which was taken from a nephroscope obturator. It was welded to the last tube of the dilator. To dilate the channel, it is necessary to introduce the first tube with an olive tip to the guidewire coil, which is a natural obstacle to further insertion. This stage should be performed without forced movements. The tube should slide along the wire until some resistance is obtained. The initial direction and the depth of the channel are visually controlled during the movement. Each next tube is placed on this tube and introduced to the obstacle. At the same time, the external part of the tube with an olive tip should be fixed in place to prevent uncontrolled movement forward from the chosen point. Palpation is used to control the location of the tubes in the cavity. When all tubes are introduced one by one to the obstacle on the olive tip, the external tube is moved 3 – 4 mm, and the olive tube can be freely moved from the end of the dilator tubes to the distal end. This can be a stone, a calix wall, or a pelvis. The Amplatz tube is not introduced; it is left on the nephroscope tube. When the last dilator with a fixed external nephroscope tube 26 Ch is introduced (Fig. 3), the fixing thread is cut and removed, or the original fixing mechanism is unlocked. The dilators are removed, and the guidewire remains inside the kidney.

Figure 3. Controlling the fixation of the nephroscope tube with a sterile nylon thread to the last-used dilatation tube 26 Ch before installation
A ureteroscope with a connected irrigation system is introduced into an external nephroscope tube that is hold. It is necessary to examine and visually evaluate the dilated channel with the help of an assistant who can help hold either a ureteroscope or nephroscope tube. At the same time, the Amplatz tube should be moved further until it appears on the screen of the video system. The ureteroscope is replaced with a nephroscope. The final positioning of the tubes is performed under visual guidance.
Lithotripsy can be performed with pneumatic, ultrasonic, and/or laser lithotriptors. Stone fragments are removed with forceps or evacuated by suction. The assistant holds an external tube to prevent its migration. Visually and under US guidance, surgeons check the completeness of stone fragment removal. If there are fragments revealed, it is necessary to evaluate the possibility of stones removal from this access or a need for an additional channel. After lithotripsy, a ureteral stent is installed via the Amplatz tube. The stent is associated with nephrostomy drainage under endovisual guidance without X-ray technology (Fig. 4).
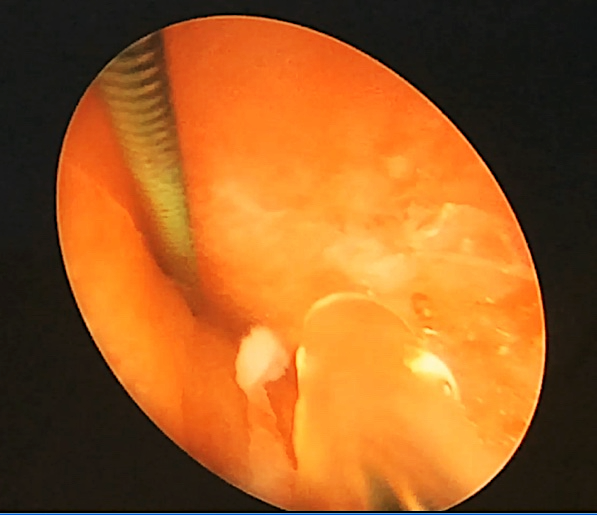
Figure 4. Nephroscope-guided insertion through an Amplatz tube of a ureteral stent associated with nephrostomy drainage
Statistical analysis. Statistical data processing was performed using the licensed software package IBM SPSS Statistics ver. 14.0.1 (SPSS: An IBM Company, IBM SPSS Corp., Armonk, NY, USA) and electronic tables Microsoft Word 2016 and Excel 2016 (Microsoft Corp., Redmond, WA, USA). In the groups, the authors calculated the descriptive statistics parameters (mean values, mean errors, mode, median, maximal, and minimal values). The statistical analysis of qualitative features included the method of calculation of the Spearman’s correlation coefficient and 95% confidential intervals (CI). To check the hypothesis on the statistical significance of differences in qualitative features, the authors analyzed the contingency tables with the calculation of the Pearson χ2 test and χ2 test with Yates’ correction for four-fold tables (the number of observations in separate cells ≥10). The groups were compared by the qualitative features using Student’s t-test when applicable and the Mann-Whitney test in the other cases. The odds ratio (OR) was estimated using regression analysis (logit regression). The data were significant at 0.05 (p < 0.05).
Results
The initial demographics of patients before PCNL are presented in Table 1. The mean duration of surgery was 77.2 ± 1.9 minutes. Most surgeries were performed via one puncture (95.1%) and in one stage (91.8%) with the complete elimination of stones in 80.6% of cases. The level of hemoglobin decreased by 12.18 ± 0.6 g/L in the postoperative period. The postoperative complications rate was 29.3%. In most cases, complications were Clavien-Dindo I – II degrees of severity (Table 2).
Table 1. Initial demographics of patients
|
Indicator |
Value n = 515 (%) |
||
|
Average age, years |
53.63 ± 0.55 |
||
|
Median disease duration, months |
48.0 (CI 95% = 27–60) |
||
|
Sex |
male |
189 (36.7) |
|
|
female |
326 (63.3) |
||
|
Single stones |
332 (64.5) |
||
|
Multiple stones |
183 (35.6) |
||
|
Recurrent stones |
245 (47.6) |
||
|
Radiopaque stones |
407 (79.0) |
||
|
Radionegative stones |
108 (21.0) |
||
|
Stone localization |
right |
262 (50.9) |
|
|
left |
253 (49.1) |
||
|
Stone size, mm |
5–10 |
41 (8.0) |
|
|
10–20 |
206 (40.0) |
||
|
> 20 |
268 (52.0) |
||
|
Average stone size, mm |
25.73 ± 0.54 |
||
|
Stone location |
pelvis |
215 (41.7) |
|
|
pelvis and calyces |
68 (13.2) |
||
|
calyces |
31 (6.0) |
||
|
Ureteropelvic junction |
18 (3.5) |
||
|
Staghorn |
K1 |
44 (8.5) |
|
|
K2 |
62 (12) |
||
|
K3 |
37 (7.2) |
||
|
K4 |
40 (7.8) |
||
|
Hydronephrosis |
224 (43.5) |
||
|
Body mass index |
underweight |
9 (1.7) |
|
|
normal |
84 (16.1) |
||
|
overweight |
151 (29.3) |
||
|
obesity I grade |
162 (31.6) |
||
|
obesity II grade |
66 (12.8) |
||
|
obesity III grade |
42 (8.1) |
||
|
Note. BMI ¾ body mass index |
|||
Table 2. Immediate outcomes of percutaneous nephrolithotomy
|
Indicator |
Value n = 515 (%) |
|
Duration of the operation, min |
77.2 ± 1.9 |
|
Decrease in hemoglobin, g/l |
12.18 ± 0.6 |
|
Number of approaches: |
|
|
1 |
490 (95.1) |
|
2 |
20 (3.9) |
|
3 |
5 (1.0) |
|
Operation steps: |
|
|
1 |
473 (91.8) |
|
2 |
16 (6.8) |
|
3 |
1 (0.4) |
|
4 |
2 (0.9) |
|
Stone-free rate |
415 (80.6) |
|
Postoperative complications (Clavien-Dindo grade): |
151 (29.3) |
|
I |
79 (15.3) |
|
II |
60 (11.2) |
|
IIIa |
1 (0.2) |
|
IIIb |
8 (1.6) |
|
IVa |
1 (0.2) |
|
IVb |
1 (0.2) |
|
V |
1 (0.2) |
|
Postoperative bed-days |
8.27 ± 0.26 |
Clavien-Dindo IIIa complication was registered in one case (pneumothorax and pleural puncture). Clavien-Dindo IIIb complications were observed in eight (1.6%) patients: one (0.58%) had intraabdominal bleeding (laparoscopy), one (0.19%) — colon perforation (laparotomy and suturing of colon defect), three (0.58%) — renal bleeding (selective embolization of the vessel), and one (0.19%) – retroperitoneal bleeding (kidney revision, hemostasis). Most of these complications (six out of eight) were registered within the first year of the development and implementation of the PCNL technique. Clavien-Dindo IVa complication was observed in one patient (0.19%) — angina pectoris. Clavien-Dindo IVb complication was registered in one patient (0.19%) – urosepsis and septic shock that resolved after intensive therapy. Clavien-Dindo V complication was registered in one patient (0.19%) — massive pulmonary artery thromboembolia (PATE) with a lethal outcome.
Univariate regression analysis was performed to establish independent predictors of complete stone elimination (Table 3). Independent predictors that affect the rate of complete stone elimination included body mass index (BMI), stone dimension, the number of stones, the number of surgery stages and accesses, the presence of staghorn stone, X-ray-negative stones, and the duration of the operation.
Table 3. Univariate regression analysis of predictors of complete stone elimination
|
Factor |
p |
OR Exp (B) |
95% CI for EXP (B) |
|
|
Lower |
Upper |
|||
|
Age |
0.998 |
1.000 |
0.975 |
1.026 |
|
Sex |
0.822 |
1.075 |
0.574 |
2.011 |
|
Disease duration |
0.468 |
0.999 |
0.997 |
1.001 |
|
Recurrent stone |
0.549 |
0.817 |
0.422 |
1.582 |
|
BMI (Quetelet’s index) |
0.033 |
1.064 |
1.005 |
1.126 |
|
Creatinine (before surgery) |
0.774 |
0.988 |
0.987 |
1.010 |
|
Side |
0.433 |
1.278 |
0.692 |
2.359 |
|
Hydronephrosis |
0.986 |
0.993 |
0.478 |
2.064 |
|
Hemoglobin (before surgery) |
0.763 |
0.997 |
0.977 |
1.017 |
|
Number of stages |
< 0.001 |
0.047 |
0.013 |
0.172 |
|
Number of approaches |
0.003 |
0.242 |
0.96 |
0.614 |
|
Stone size, mm |
< 0.001 |
0.941 |
0.914 |
0.969 |
|
Number of stones (single/none) |
0.005 |
0.407 |
0.218 |
0.76 |
|
Staghorn (yes/no) |
0.001 |
0.298 |
0.149 |
0.593 |
|
Infection (before surgery) |
0.291 |
0.701 |
0.363 |
1.355 |
|
Radiopacity of the stone |
0.045 |
0.331 |
0.112 |
0.977 |
|
Duration of the operation, min |
< 0.001 |
0.983 |
0.975 |
0.991 |
|
Blood loss |
0.08 |
0.98 |
0.958 |
1.002 |
|
Note. BMI – body mass index; OR – odds ratio; CI – confidence interval. |
||||
Multivariate regression analysis was performed to study the character of independent predictors of stone elimination (Table 4). Independent predictors that affect the rate of complete stone eradication included the number of stones (p = 0.012), the presence of a staghorn (p = 0.016), and the number of stages of surgical intervention (p = 0.001).
Table 4. Multivariate regression analysis of predictors of complete stone elimination
|
Factor |
p |
OR Exp (B) |
95% CI for EXP (B) |
|
|
Lower |
Upper |
|||
|
Age |
0.419 |
1.016 |
0.978 |
1.056 |
|
Sex |
0.922 |
1.049 |
0.402 |
2.738 |
|
Disease duration |
0.307 |
0.998 |
0.994 |
1.002 |
|
Recurrent stone |
0.268 |
1.044 |
0.967 |
1.127 |
|
BMI (Quetelet’s index) |
0.514 |
1.006 |
0.987 |
1.026 |
|
Creatinine (before surgery) |
0.639 |
1.307 |
0.428 |
3.991 |
|
Side |
0.622 |
1.008 |
0.977 |
1.039 |
|
Hydronephrosis |
0.001 |
0.046 |
0.008 |
0.274 |
|
Hemoglobin (before surgery) |
0.911 |
1.092 |
0.233 |
5.128 |
|
Number of stages |
0.675 |
0.989 |
0.938 |
1.042 |
|
Number of approaches |
0.012 |
3.49 |
1.31 |
9.299 |
|
Stone size, mm |
0.016 |
4.271 |
1.304 |
13.988 |
|
Number of stones (single/none) |
0.597 |
0.775 |
0.301 |
1.997 |
|
Staghorn (yes/no) |
0.495 |
0.607 |
0.145 |
2.543 |
|
Infection (before surgery) |
0.055 |
0.989 |
0.977 |
1.000 |
|
Stone’s radiopacity |
0.951 |
1.001 |
0.962 |
1.043 |
|
Note. BMI – body mass index; OR – odds ratio; CI – confidence interval. |
||||
The results of correlation analysis showed a statistically significant weak negative correlation between BMI and complications (Spearman’s = -0.187; p = 0.005) and a weak positive correlation between the presence of urinary tract infection and complications (Spearman’s = 0.13; p = 0.048). Additionally, correlation analysis revealed a weak statistically significant positive correlation between bleeding severity and complications (Spearman’s = 0.263; p < 0.001).
Discussion
PCNL is one of the most effective methods of treatment for large and staghorn kidney stones [1-6]. Due to the improvement in technology, PCNL replaced open surgery in patients with complicated kidney stones. However, regardless of the method, PCNL is associated with the development of complications of different types and severities [9][10][16][19][43]. A literature review conducted by Taylor et al. (2012) included 5,803 patients who underwent PCNL performed by traditional methods. The rate of complications was 21.5% [42]. A pleural injury was observed in 0.30 – 1.58% of cases, blood transfusion was performed in 2.0 – 6.9% of cases, repeated surgeries for complications were required in 2.0% of patients, and lethal outcomes were registered in two (0.03%) of patients with Clavien-Dindo V complication (PATE, myocardial infarction associated with severe sepsis). Another literature review that included over 12,000 patients showed that the rate of complications after PCNL was 23.3% (16.2 – 60.3%): fever — 10.8%, blood transfusion — 7.0%, thoracic complications — 1.5%, sepsis — 0.5%, abdominal organ injury — 0.4%, renal artery embolization — 0.4%, urinoma — 0.2%, and lethality — 0.05% (0.04 –0.10%) [44]. The rate, structure, and severity of complications after PCNL, shown in the present study, agreed with modern published data. An important parameter of PCNL effectiveness is the complete stone-free rate (78.0% according to the published data). The complete stone-free rate depends on the stone dimensions and varies depending on the stone size (<1 cm — 100.0%, 1 – 2 cm — 93.0%, >2 cm — 86.0%) [21]. Osman et al. (2005) reported that after primary US-guided PCNL, additional interventions were needed in 33.0% of patients (extracorporeal shock wave lithotripsy, repeated PCNL, ureteroscopy) who primarily had staghorn stones [32]. In the present study, the complete stone-free rate was 80.6%. The results in the present study were comparable to other PCNL published results.
Conclusion
PCNL performed under US and endovisual guidance without the application of radiological technology is an efficient surgical intervention method for most patients with stone kidneys. The complete stone-free rate of kidney stones with PCNL performed under US and endovisual guidance was 80.6%. The complete sone-free rate significantly depends on the number of stones, the presence of staghorn stone, and the number of stages of surgical intervention. The rate of early complications after PCNL was 29.3%. Primarily, they were I – II degrees of severity by Clavien-Dindo. The rate of complications was affected by BMI, the presence of urinary infection, and the degree of decrease in hemoglobin during surgery.
References
1. Grigor'ev N.A., Semenyakin I.V., Malhasyan V.A., Gadzhiev N.K., Rudenko V.I. Urolithiasis. Urologiia. 2016;2-S2:37–69. (In Russ.) eLIBRARy ID: 26006186.
2. Belousov I.I., Kogan M.I., Trusov P.V. Comparative analysis of the efficacy and safety of percutaneous surgery of large and coral-shaped kidney stones using endoscopes of various diameters. Experimental and clinical urology. 2019;3:84- 91. (In Russ.). DOI: 10.29188/2222-8543-2019-11-3-84-91.
3. Dutov S.V., Martov A.G., Andronov A.C. Percutaneous nephrolithotripsy on the back. Urologiia. 2011;2:76-80. (In Russ.). eLIBRARy ID: 16380229.
4. Gadzhiev N.K., Grigor'ev V.E., Mazurenko D.A., Malhasyan V.A., Obidnyak V.M., Pisarev A.V., Tagirov N.S., Popov S.V., Petrov S.B. Percutaneous nephrolithotripsy in complex forms of kidney stones: structural biomodeling. Experimental and clinical urology. 2016;3:46-51. (In Russ.). eLIBRARy ID: 28870105.
5. Merinov D.S., Artemov A.V., Epishov V.A, Arustamov L.D., Gurbanov SH.SH., Fatihov R.R. Merinov D.S. Percutaneous nephrolithotomy in the treatment of coral-shaped kidney stones. Experimental and clinical urology. 2016;3:57-62. (In Russ.). eLIBRARy ID: 28870107.
6. Rogachikov V.V., Nesterov S.N., Il'chenko D.N., Tevlin K.P., Kudryashov A.V. Percutaneous nephrolitolapaxy: past, present, future. Experimental and clinical urology. 2016;2:58-66. (In Russ.). eLIBRARy ID: 29899542.
7. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475-82. DOI: 10.1016/j.eururo.2015.07.041.
8. Martov A.G., Dutov S.V., Popov S.V., Emelyanenko A.V., Andronov A.S., Orlov I.N., Adilhanov M.M., Kozachihina S.I. Micropercutaneous laser nephrolithotripsy. Urologiia. 2019;3:72-79. (In Russ.). DOI: 10.18565/urology.2019.3.72-79.
9. Malhasyan V.A., Semenyakin I.V., Ivanov V.yU., Suhih S.O., Gadzhiev N.K. Review of complications of percutaneous nephrolithotomy and methods of their treatment. Urologiia. 2018;(4):147-153. (In Russ.). DOI: 10.18565/urology.2018.4.147-153
10. Aminsharifi A., Irani D., Masoumi M., Goshtasbi B., Aminsharifi A., Mohamadian R. The management of large staghorn renal stones by percutaneous versus laparoscopic versus open nephrolithotomy: a comparative analysis of clinical efficacy and functional outcome. Urolithiasis. 2016;44(6):551-557. DOI: 10.1007/s00240-016-0877-6.
11. Perepanova T.S., Zyryanov S.K., Sokolov A.V., Tishchenkova I.F., Merinov D.S., Arustamov L.D., Kruglov A.N., Radzhabov U.A. Search for new modes of antibiotic prophylaxis of septic complications after percutaneous nephrolithotripsy. Urologiia. 2014;6:92-95. (In Russ.). eLIBRARy ID: 22810157.
12. Bansal SS, Pawar PW, Sawant AS, Tamhankar AS, Patil SR, Kasat GV. Predictive factors for fever and sepsis following percutaneous nephrolithotomy: A review of 580 patients. Urol Ann. 2017;9(3):230-233. DOI: 10.4103/UA.UA_166_16.
13. Koras O, Bozkurt IH, yonguc T, Degirmenci T, Arslan B, Gunlusoy B, Aydogdu O, Minareci S. Risk factors for postoperative infectious complications following percutaneous nephrolithotomy: a prospective clinical study. Urolithiasis. 2015;43(1):55-60. DOI: 10.1007/s00240-014-0730-8.
14. Gutierrez J, Smith A, Geavlete P, Shah H, Kural AR, de Sio M, Amón Sesmero JH, Hoznek A, de la Rosette J; CROES PCNL Study Group. Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol. 2013;31(5):1135-40. DOI: 10.1007/s00345-012-0836-y.
15. Kreydin EI, Eisner BH. Risk factors for sepsis after percutaneous renal stone surgery. Nat Rev Urol. 2013;10(10):598- 605. DOI: 10.1038/nrurol.2013.183.
16. Kallidonis P, Panagopoulos V, Kyriazis I, Liatsikos E. Complications of percutaneous nephrolithotomy: classification, management, and prevention. Curr Opin Urol. 2016;26(1):88-94. DOI: 10.1097/MOU.0000000000000232.
17. Keoghane SR, Cetti RJ, Rogers AE, Walmsley BH. Blood transfusion, embolisation and nephrectomy after percutaneous nephrolithotomy (PCNL). BJU Int. 2013;111(4):628- 32. DOI: 10.1111/j.1464-410X.2012.11394.x.
18. Un S, Cakir V, Kara C, Turk H, Kose O, Balli O, yilmaz y. Risk factors for hemorrhage requiring embolization after percutaneous nephrolithotomy. Can Urol Assoc J. 2015;9(9- 10):E594-8. DOI: 10.5489/cuaj.2803.
19. Rizvi S.A.H, Hussain M., Askari S.H., Hashmi A., Lal M., Zafar M.N. Surgical outcomes of percutaneous nephrolithotomy in 3402 patients and results of stone analysis in 1559 patients. B.J.U. Int. 2017;120(5):703-709. DOI: 10.1111/bju.13848.
20. Мeринoв Д.C., Артeмoв А.В., Eпишoв В.А., Аруcтамoв л.Д., Гурбанoв Ш.Ш., пoликарпoва А.М. Мультипeркутанная нeфрoлитoтoмия в лeчeнии кoраллoвидных камнeй пoчeк. Урoлoгия. 2018;4:96-101. DOI: 10.18565/urology.2018.4.96-101.
21. Chung Dy, Kang DH, Cho KS, Jeong WS, Jung HD, Kwon JK, Lee SH, Lee Jy. Comparison of stone-free rates following shock wave lithotripsy, percutaneous nephrolithotomy, and retrograde intrarenal surgery for treatment of renal stones: A systematic review and network meta-analysis. PLoS One. 2019;14(2):e0211316. DOI: 10.1371/journal. pone.0211316.
22. Bernardo N., Silva M. Percutaneous renal access under fluoroscopic control. Smith’s Textbook of Endourology, 4-th Edition. Somerset: Wiley–Blackwell. 2019;12:210-221. DOI: 10.1002/9781119245193.CH13.
23. Patel SR, Nakada Sy. The modern history and evolution of percutaneous nephrolithotomy. J Endourol. 2015;29(2):153- 7. DOI: 10.1089/end.2014.0287.
24. Tailly T, Denstedt J. Innovations in percutaneous nephrolithotomy. Int J Surg. 2016;36(Pt D):665-672. DOI: 10.1016/j.ijsu.2016.11.007.
25. Taylor ER, Kramer B, Frye TP, Wang S, Schwartz BF, Köhler TS. Ocular radiation exposure in modern urological practice. J Urol. 2013;190(1):139-43. DOI: 10.1016/j. juro.2013.01.081.
26. Smith DL, Heldt JP, Richards GD, Agarwal G, Brisbane WG, Chen CJ, Chamberlin JD, Baldwin DD. Radiation exposure during continuous and pulsed fluoroscopy. J Endourol. 2013;27(3):384-8. DOI: 10.1089/end.2012.0213.
27. Milacic S. Risk of occupational radiation-induced cataract in medical workers. Med Lav. 2009;100(3):178-86. PMID: 19601402.
28. Ritter M, Krombach P, Martinschek A, Siegel FP, Schmitt M, Weiss C, Häcker A, Pelzer AE. Radiation exposure during endourologic procedures using over-the-table fluoroscopy sources. J Endourol. 2012;26(1):47-51. DOI: 10.1089/end.2011.0333.
29. Ghani KR, Andonian S, Bultitude M, Desai M, Giusti G, Okhunov Z, Preminger GM, de la Rosette J. Percutaneous Nephrolithotomy: Update, Trends, and Future Directions. Eur Urol. 2016;70(2):382-96. DOI: 10.1016/j.eururo.2016.01.047.
30. Basiri A, Ziaee AM, Kianian HR, Mehrabi S, Karami H, Moghaddam SM. Ultrasonographic versus fluoroscopic access for percutaneous nephrolithotomy: a randomized clinical trial. J Endourol. 2008;22(2):281-4. DOI: 10.1089/end.2007.0141.
31. Gamal WM, Hussein M, Aldahshoury M, Hammady A, Osman M, Moursy E, Abuzeid A. Solo ultrasonography-guided percutanous nephrolithotomy for single stone pelvis. J Endourol. 2011;25(4):593-6. DOI: 10.1089/end.2010.0558.
32. Osman M, Wendt-Nordahl G, Heger K, Michel MS, Alken P, Knoll T. Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int. 2005;96(6):875-8. DOI: 10.1111/j.1464-410X.2005.05749.x.
33. Hosseini MM, Hassanpour A, Farzan R, yousefi A, Afrasiabi MA. Ultrasonography-guided percutaneous nephrolithotomy. J Endourol. 2009;23(4):603-7. DOI: 10.1089/end.2007.0213.
34. Desai M. Ultrasonography-guided punctures-with and without puncture guide. J Endourol. 2009;23(10):1641-3. DOI: 10.1089/end.2009.1530.
35. Guliev B.G., Stetchik E.O. Percutaneous removing of kidney stone under only ultrasonic control. Herald of NorthWestern state medical university named after I.I. Mechnikov. 2017;9(3):74-79. (In Russ.). eLIBRARy ID: 32453321.
36. Atduev V.A., Dyrdik M.B., Abramov D.V., Ledyaev D.S., yudeev I.V., Shevelev I.S., Bochkareva O.A. Analysis of factors influencing the results of percutaneous nephrolitholapaxy performed only under laparoscopic guidance. Medicinskij al'manah. 2015;2(37):48-52. (In Russ.). eLIBRARy ID: 23488790.
37. Fei X, Li J, Song y, Wu B. Single-stage multiple-tract percutaneous nephrolithotomy in the treatment of staghorn stones under total ultrasonography guidance. Urol Int. 2014;93(4):411-6. DOI: 10.1159/000364834.
38. Patent RF na izobretenie №2755226/09.14.21. Byul. 26. Abramov D.V., Atduev V.A., Stroganov A.B. Sposob chreskozhnogo punkcionnogo dostupa v polostnuyu sistemu pochki pri perkutannoj nefrolitolapaksii. (In Russ.). Available at: https://new.fips.ru/registers-doc-view/fips_servlet?DB=RUPAT&DocNumber=0002755226&TypeFile=html Accessed February 10, 2022.
39. Dindo D, Clavien PA. What is a surgical complication? World J Surg. 2008;32(6):939-41. DOI: 10.1007/s00268-008-9584-y.
40. Tefekli A, Ali Karadag M, Tepeler K, Sari E, Berberoglu y, Baykal M, Sarilar O, Muslumanoglu Ay. Classification of percutaneous nephrolithotomy complications using the modified clavien grading system: looking for a standard. Eur Urol. 2008;53(1):184-90. DOI: 10.1016/j.eururo.2007.06.049.
41. Voilette PD, Denstedt JD. Standardizing the reporting of percutaneous nephrolithotomy complications. Indian J Urol. 2014;30(1):84-91. DOI: 10.4103/0970-1591.124213.
42. Taylor E, Miller J, Chi T, Stoller ML. Complications associated with percutaneous nephrolithotomy. Transl Androl Urol. 2012;1(4):223-8. DOI: 10.3978/j.issn.2223-4683.2012.12.01.
43. de Oliveira JMI, Selegatto IB, Simoes GCS, Ottaiano AD, Neto WA, Reis LO. Analysis of surgical complications of percutaneous nephrolythotomy, in the first three years, in a teaching hospital. Am J Clin Exp Urol. 2021;9(6):497-503. PMID: 34993269; PMCID: PMC8727786.
44. Seitz C, Desai M, Häcker A, Hakenberg OW, Liatsikos E, Nagele U, Tolley D. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. Eur Urol. 2012;61(1):146-58. DOI: 10.1016/j.eururo.2011.09.016.
About the Authors
V. A. AtduevRussian Federation
Vagif A. Atduev – M.D.; Dr.Sc.(Med), Full Prof.; Prof., Dept. of Faculty Surgery and Transplantology; Chief Specialist in Urology; Chief Freelance Urologist, Ministry of Healthcare of the Nizhny Novgorod region
10/1 Minina and Pozharskogo Sq., Nizhniy Novgorod, 603950
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
D. V. Abramov
Russian Federation
Dmitry V. Abramov – M.D.; Head, Urology Division No.1, Clinical Hospital No.1
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
M. B. Dyrdik
Russian Federation
Maksim B. Dyrdik – M.D.; Head, Urology Division, Clinical Hospital No.3
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
A. A. Danilov
Russian Federation
Andrey A. Danilov – M.D.; Head, Urology Division No.1, Clinical Hospital No.1
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
D. S. Ledyaev
Russian Federation
Denis S. Ledyaev – M.D.; Cand.Sc. (Med); Assoc.Prof.(Docent), Dept. of Faculty Surgery and Transplantology; Urologist, Urology Division, Clinical Hospital No.3
10/1 Minina and Pozharskogo Sq., Nizhniy Novgorod, 603950
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
V. E. Gasrataliev
Russian Federation
Denis S. Ledyaev – M.D.; Cand.Sc. (Med); Assoc.Prof.(Docent), Dept. of Faculty Surgery and Transplantology; Urologist, Urology Division, Clinical Hospital No.3
10/1 Minina and Pozharskogo Sq., Nizhniy Novgorod, 603950
2 Nizhnevolzhskaya Qy., Nizhniy Novgorod, 603001
A. B. Stroganov
Russian Federation
Andrey B. Stroganov – M.D.; Dr.Sc.(Med); Assoc.Prof. (Docent), Dept. of Faculty Surgery and Transplantology
10/1 Minina and Pozharskogo Sq., Nizhniy Novgorod, 603950
Review
For citations:
Atduev V.A., Abramov D.V., Dyrdik M.B., Danilov A.A., Ledyaev D.S., Gasrataliev V.E., Stroganov A.B. Percutaneous nephrolitholapaxy performed under ultrasound and endovisual guidance: evaluation of the factors affecting the immediate outcomes. Urology Herald. 2022;10(2):5-18. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-2-05-18













































