Scroll to:
Optimizing compliance and efficacy in intravesical BCG therapy for non-muscle invasive bladder cancer: tailored strategies and clinical outcomes
https://doi.org/10.21886/2308-6424-2024-12-6-76-82
Abstract
Introduction. Bladder cancer ranks among the most prevalent malignancies worldwide, with non-muscle invasive bladder cancer (NMIBC) constituting most cases. Intravesical Bacillus Calmette-Guérin (BCG) therapy is a cornerstone in NMIBC management, reducing recurrence and progression rates. However, patient compliance remains a challenge due to adverse effects, discomfort, and the demanding treatment regimen.
Objective. To evaluate the tolerability and complications associated with intravesical BCG therapy and to assess the efficacy of strategies implemented to enhance patient compliance and mitigate dropout rates.
Materials & Methods. A retrospective analysis was conducted on 163 patients diagnosed with NMIBC who underwent intravesical BCG therapy at our institution from August 2018 to December 2022. Data included patient demographics, tumour stage, BCG dosage, therapy duration, and adverse effects. Compliance strategies involved dedicated outpatient clinics, exclusive resident and nursing staff, and proactive toxicity management. Dosage modulation to 80 mg was employed for patients unable to tolerate the standard 120 mg BCG dose.
Results. Among the cohort, 86 % were male, with 63 % being chronic smokers. Recurrence occurred in 16 % of patients, with 4 progressing to muscle-invasive disease (T2). BCG-related toxicity (Grade 1) was observed in 50 % of patients, with conservative management proving effective. A dose reduction to 80 mg was necessary in 17 % of patients, improving tolerance in the majority. Key compliance strategies significantly reduced dropout rates, with 80 % of patients completing the full course of therapy.
Conclusion. Strategic interventions such as dosage modulation, dedicated clinic days, and comprehensive patient counselling markedly improved adherence to intravesical BCG therapy, reducing adverse effects and dropout rates. These findings underscore the importance of tailored strategies to enhance compliance, ultimately improving NMIBC management and patient outcomes.
Keywords
For citations:
Shubhankar G., Mittal A., Panwar V.K., Ghorai R.P. Optimizing compliance and efficacy in intravesical BCG therapy for non-muscle invasive bladder cancer: tailored strategies and clinical outcomes. Urology Herald. 2024;12(6):76-82. https://doi.org/10.21886/2308-6424-2024-12-6-76-82
Introduction
Bladder cancer ranks as the seventh most prevalent malignancy among males and the eleventh overall globally. Approximately 70% of these cases present as non-muscle invasive bladder cancer (NMIBC), with the majority at stage Ta (confined to the mucosa), 20% at stage T1 (limited to the submucosa), and 10% classified as carcinoma in situ (CIS). Recurrence afflicts 50% to 70% of low-grade Ta lesions, with 5% progressing to more invasive stages. In high-grade T1 lesions, the recurrence rate exceeds 80% within three years, with progression occurring in 50% of cases [1]. Intravesical Bacillus Calmette-Guérin (BCG) therapy is a cornerstone of NMIBC management, significantly curtailing both recurrence and progression rates. However, its efficacy in practice is critically dependent on patient compliance with the demanding treatment regimen and the tolerance of its adverse effects. Dropout rates in BCG therapy vary widely, typically ranging from 10% to 30%, largely due to adverse effects, patient discomfort in busy outpatient settings, and the stringent treatment schedule [2]. Addressing these compliance issues at a foundational level is imperative. This study undertook a retrospective observational approach at a single institution to scrutinise the tolerability and complications associated with intravesical BCG therapy, while concurrently evaluating the efficacy of targeted strategies aimed at optimising patient adherence.
Objective. This study aimed to evaluate the tolerability and complications associated with intravesical BCG therapy and to assess the efficacy of strategies implemented to enhance patient compliance and mitigate dropout rates.
Materials & methods
It was a retrospective cohort study. Informed consent was meticulously obtained from all participants enrolled in this study, with ethical approval secured from the Institutional Ethics Committee (IEC) before its initiation (AIIMS/IEC/21/545, date of approval 22/10/2021). The inclusion criteria were all the NMIBC patients who were planned for intravesical BCG therapy. The NMIBC patients with variant histology, endoscopically unmanageable cases or those not giving consent for the intravesical therapy were excluded from the study. The medical records of all patients diagnosed with NMIBC who underwent intravesical BCG therapy at our institution from August 2018 to December 2022 were retrospectively aggregated and meticulously scrutinised. Patients included in the study received 6 weekly doses of intravesical BCG followed by 12 monthly doses. This BCG regimen was supplemented with a 3 monthly check cystoscopy with urine cytology to assess for any recurrence throughout treatment. The Danish 1331 strain-derived BCG vaccine was used. Data extraction encompassed variables such as age, sex, tumour stage, histopathological findings, urinalysis (routine, microscopy, culture, and sensitivity), intravesical BCG dosage, and the duration of both induction and maintenance BCG therapy. Figure 1 presents the study algorithm. Special attention was given to the assessment of BCG-related adverse effects and the strategies implemented to mitigate dropout rates and enhance patient adherence. Compliance-enhancing measures included the establishment of dedicated outpatient clinic days, the assignment of exclusive resident and nursing staff, meticulous maintenance of BCG registers, and proactive management of BCG toxicity and intolerance.
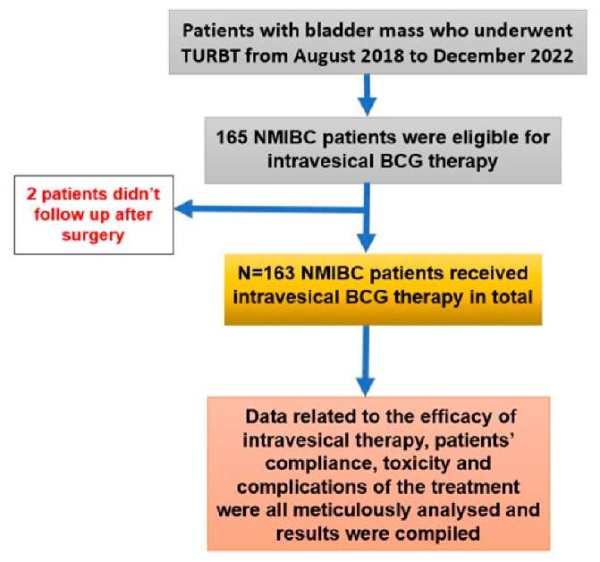
Figure 1. Study algorithm
Statistics. Data analysis was done using IBM SPSS Statistics ver. 28.0 («SPSS: An IBM Company», IBM SPSS Corp., Armonk, NY, USA). Data are presented as absolute (n) and relative values — percentage and frequency (%).
Results
Among the 163 patients who received intravesical BCG therapy during the study period, a substantial majority of 86% (148) were male, while 14% (23) were female. The mean age of the patients was 54 ± 17 years. Comorbidities were prevalent, with hypertension in 26%, diabetes mellitus in 13%, and chronic kidney disease in 5% of the cohort. Additionally, 104 patients (63%) were chronic smokers, with a significant gender disparity (101 males and 3 females). Histopathological examination revealed that 50 patients (30%) had T1 low-grade carcinoma, 109 patients (67%) had T1 high-grade carcinoma, and 4 patients (3%) had Ta low-grade disease. Figure 2 presents the proportion of patients with T1HG, T1LG and Ta LG tumours.
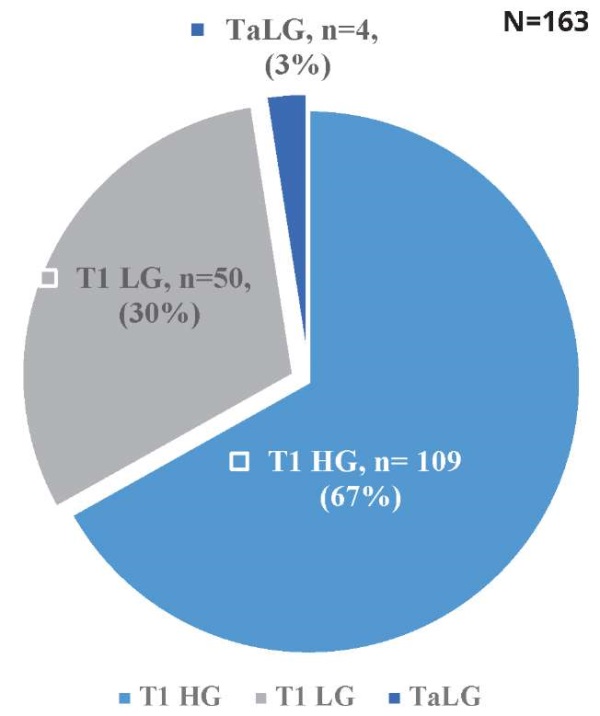
Figure 2. The proportion of patients with T1HG, T1LG and TaLG tumours
Of the cohort, 131 patients completed the induction and maintenance courses successfully. The remaining 32 patients experienced recurrence, exhibited BCG intolerance, defaulted, or otherwise discontinued therapy. Specifically, 29 patients (17%) could not tolerate the 120 mg dose, necessitating a reduction to 80 mg. Post dose reduction, 26 of these 29 patients could complete their BCG therapy at the lower dose, while intravesical BCG was discontinued in 3 patients due to intolerance even at the 80 mg dosage.
Two patients discontinued intravesical BCG therapy, one during the induction phase and another during the mid-maintenance phase, due to unforeseen familial tragedies and relocation, respectively.
A total of 82 patients (50%) experienced Grade 1 BCG toxicity, which was managed conservatively with antibiotics, anticholinergic medications, and Pyridium. Five patients (3%) encountered Grade 2 BCG toxicity, and three patients (1%) experienced Grade 3 BCG toxicity, necessitating the initiation of anti-tubercular treatment. Figure 3 presents the data on BCG toxicity.
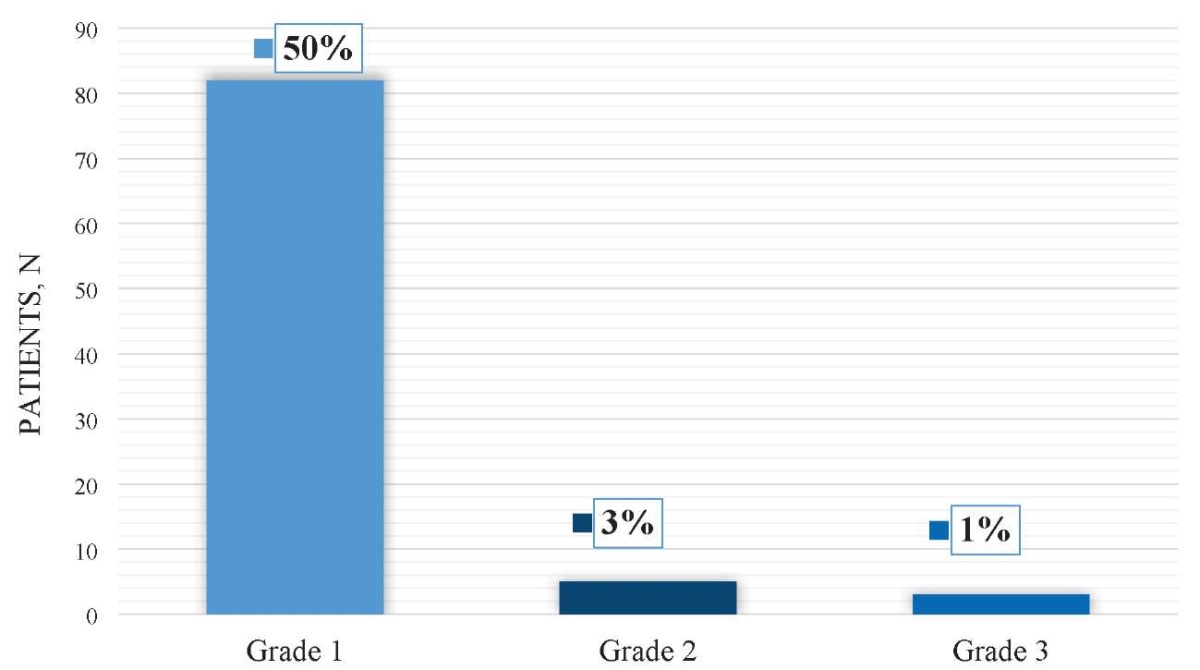
Figure 3. BCG toxicity grades
Regarding symptomatic adverse events, 82 patients (50%) reported irritative lower urinary tract symptoms (LUTS), 49 patients (30%) had urinary tract infections (UTIs), 44 patients (27%) experienced fever, and 48 patients (28%) had hematuria. Additionally, bladder contracture was observed in 3 patients, and epididymitis was noted in 1 patient. It was found that the incidence of LUTS and hematuria was significantly lower in patients receiving the 80 mg dosage compared to the 120 mg dosage; however, there was no significant difference in the occurrence of UTIs and fever between the two dosage groups. Figure 4 presents the data on BCG complications.
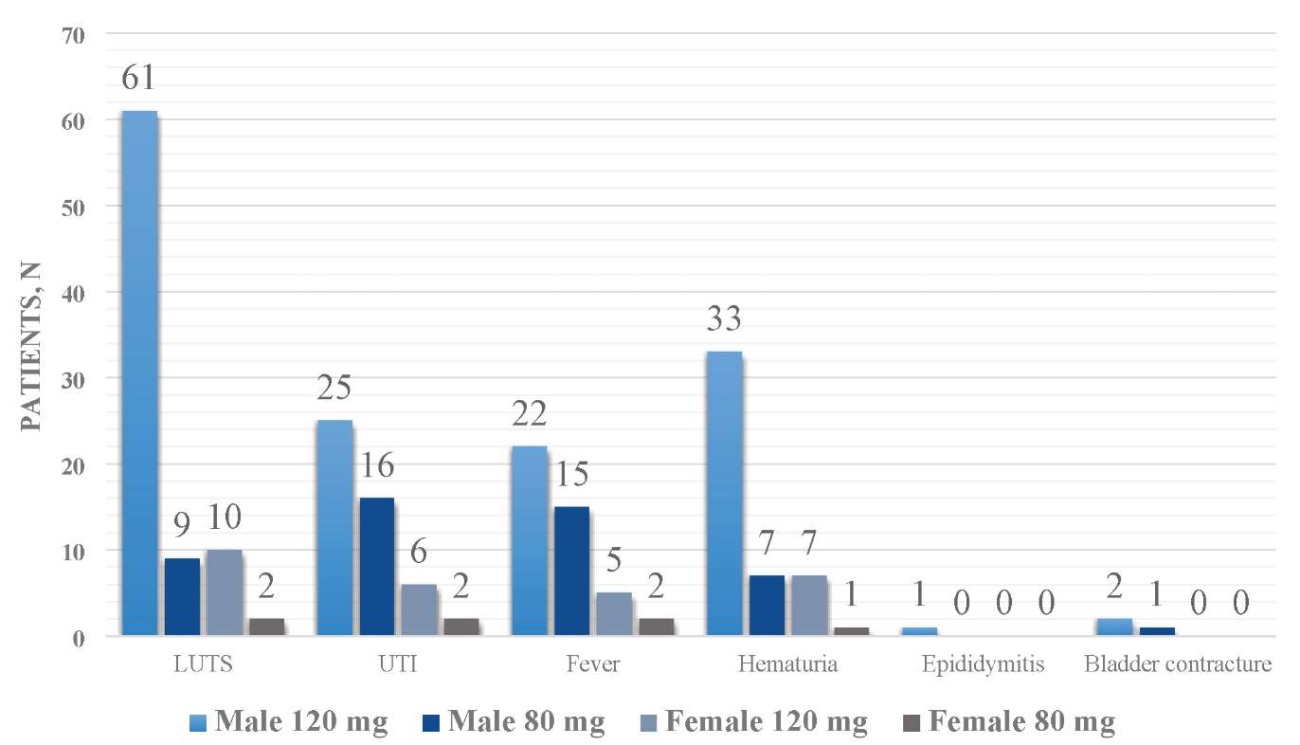
Figure 4. BCG-related dosage-depending complications
Recurrence was observed in 26 patients (16%). Among these, 4 patients (15%) progressed from T1 high grade (T1HG) to T2, 9 remained T1HG, 9 remained T1 low grade (T1LG), and 4 remained Ta low grade (TaLG).
The analysis also revealed that several stringent strategic measures were implemented to mitigate dropout rates and enhance patient adherence:
- Dedicated Outpatient Clinic Days: Specific outpatient days were designated exclusively for BCG therapy to facilitate patient convenience. Two personnel were assigned to ensure meticulous follow-up of patients scheduled on these days. These personnel, knowledgeable residents familiar with the local area, maintained contacts for economically viable accommodations, particularly aiding patients referred from other regions.
- Exclusive Resident and Nursing Staff: A dedicated resident and nursing personnel were assigned specifically for BCG therapy, maintaining direct communication with the follow-up personnel. Their responsibilities included conducting pre-therapy counselling sessions in a supportive environment, elucidating the benefits and potential adverse effects of the therapy, and emphasising the critical importance of timely follow-ups. Patients received an easily comprehensible treatment schedule and a 24/7 emergency contact number for any queries or emergencies.
- Meticulous Maintenance of BCG Registers: Comprehensive BCG registers, encompassing all pertinent patient details, were meticulously maintained in an Excel database, with regular trimonthly audits to ensure data accuracy and integrity. The follow-up personnel collaboratively conducted these audits to guarantee thorough cross-verification. They were conducted via internal monitoring.
- Proactive Management of BCG Toxicity and Intolerance: Routine follow-up calls were made to all patients on the evening of their therapy day, ensuring prompt and diligent management of any emergent complications. A trial of a modified dosage of BCG to 80 mg was given to those who couldn’t tolerate 120 mg doses before labelling them as BCG intolerant. All post-therapy complications and their subsequent management were systematically documented and graded according to the established BCG toxicity criteria. Additionally, the number of patients who discontinued therapy and the underlying reasons for their dropout were meticulously assessed.
Discussion
The current study delves into the intricacies of enhancing compliance with intravesical Bacillus Calmette-Guérin (BCG) therapy for NMIBC, underscoring the pivotal role of patient adherence in translating clinical efficacy into tangible therapeutic outcomes. This analysis aligns with the global epidemiological data delineated by Ferlay et al. (2012), which underscores the high incidence and recurrence rates associated with NMIBC, necessitating stringent compliance with therapeutic protocols [1]. The demographic profile of the cohort, predominantly male and chronic smokers, mirrors established epidemiological trends, thereby underscoring the exigency for gender-specific and lifestyle-adjusted compliance strategies. The notable prevalence of comorbidities such as hypertension, diabetes mellitus, and chronic kidney disease further accentuates the multifaceted clinical milieu that complicates NMIBC management.
BCG intolerance (as per the European Association of Urology) is defined as disease recurrence after less than adequate BCG therapy or symptomatic intolerance that mandates discontinuation of BCG therapy [3][4]. A salient finding of this investigation is the profound impact of dosage modulation on patient tolerance and adherence. The necessity to reduce the BCG dosage from 120 mg to 80 mg in 17% of patients, with subsequent successful completion of therapy in the majority, underscores the imperative for individualised treatment regimens. This is consonant with the existing literature advocating for dose adjustments to mitigate adverse effects while preserving therapeutic efficacy [5].
Toxicity from intravesical Bacillus Calmette-Guérin (BCG) therapy is classified into three grades based on severity. Mild toxicity (Grade 1) includes localised bladder irritation, mild dysuria, or increased urinary frequency; these symptoms are self-limiting and typically managed with supportive care such as analgesics or antispasmodics without altering therapy. Moderate toxicity (Grade 2) involves more pronounced cystitis symptoms like hematuria, moderate pelvic pain, or mild fever (≤ 38.5 °C). Management may include temporary cessation of BCG, dose adjustment, and symptomatic treatment such as anti-inflammatory drugs or antibiotics for secondary infections. Severe toxicity (Grade 3) presents with systemic complications like high fever (> 38.5 °C), chills, severe cystitis potentially causing bladder contracture or rare life-threatening conditions such as BCG sepsis or granulomatous prostatitis. These cases necessitate immediate cessation of therapy, systemic antituberculous treatment, and potentially hospitalisation [6][7]. This classification aids in tailoring appropriate interventions for patient safety and therapeutic efficacy. The incidence of BCG-related toxicity, predominantly Grade 1 (50%), necessitates robust management protocols to preempt and address adverse events. Conservative management with antibiotics, anticholinergics, and Pyridium proved efficacious for Grade 1 toxicities, while more severe cases required anti-tubercular treatment, underscoring the critical need for vigilant monitoring and prompt intervention [8]. The correlation between reduced dosage and lower incidence of irritative lower urinary tract symptoms (LUTS) and hematuria further substantiates the benefits of dose adjustment.
The strategic measures implemented in this study significantly mitigated dropout rates, underscoring the efficacy of targeted interventions. Dedicated outpatient clinic days, exclusive resident and nursing staff, meticulous maintenance of BCG registers, and proactive management of toxicity collectively fostered an environment conducive to adherence. Pre-therapy counselling sessions elucidating the benefits and potential adverse effects of BCG therapy were instrumental in enhancing patient understanding and commitment.
Recurrence was observed in 16% of patients, with a subset progressing to T2, reaffirming the enduring challenges in NMIBC management. This recurrence rate is consistent with the findings of Ferlay et al. (2012) and underscores the necessity for continuous surveillance and individualised therapeutic strategies to effectively manage recurrences and prevent progression [1].
In summation, this study corroborates the paramount importance of strategic compliance enhancement measures in optimising the therapeutic outcomes of intravesical BCG therapy. The correlation with referenced literature substantiates our findings and underscores the exigency for further research to refine these strategies and explore novel interventions aimed at ameliorating patient adherence and mitigating adverse effects. These strategic measures not only enhance compliance but also foster an environment conducive to achieving optimal patient outcomes in the management of NMIBC.
Limitations. This study has several limitations that must be considered. Being retrospective, it relies on existing records, potentially introducing selection bias and limiting generalisability due to the lack of randomisation. Conducted at a single centre, it may not reflect practices at other institutions. The short follow-up period only captures the immediate effects of BCG therapy, not long-term outcomes. The absence of a control group hampers comparison with alternative treatments, and variability in adverse event reporting could affect accuracy. Additionally, confounding factors such as comorbidities and inconsistent dose adjustments may influence results, while patient compliance monitoring lacked objective measurement.
Conclusion
This study highlights critical insights into the tolerability, efficacy, and compliance strategies for intravesical Bacillus Calmette-Guérin (BCG) therapy in managing non-muscle invasive bladder cancer (NMIBC). The key conclusions are:
- Patient-Centric Dosage Modulation: Reducing the BCG dose from 120 mg to 80 mg significantly improved tolerability and enabled therapy completion in the majority of patients, underscoring the importance of individualised treatment regimens.
- Impact of Strategic Compliance Measures: Implementing dedicated outpatient clinics, exclusive staff for BCG therapy, and proactive management of adverse events markedly reduced dropout rates and enhanced patient adherence, fostering optimal therapeutic outcomes.
- BCG Toxicity Management: Grade 1 toxicity was prevalent but manageable with conservative measures. More severe toxicities necessitated dose adjustments or antitubercular treatment, reinforcing the need for vigilant monitoring and tailored interventions.
- Recurrence and Progression: Recurrence was observed in 16% of patients, with a subset progressing to T2, underscoring the challenges in NMIBC management and the necessity for continuous surveillance & long-term follow-up.
Scope for Future Research. While the study underscores the efficacy of targeted interventions, limitations such as the retrospective design, single-centre data, and short follow-up period call for prospective, multicentre studies to validate these findings and explore innovative compliance-enhancement strategies. These findings emphasise the importance of integrated patient-centred approaches in optimising the outcomes of intravesical BCG therapy, paving the way for improved management of NMIBC.
References
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, eds. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. 2012. (Accessed on July 20, 2024) URL: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOB-OCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012
2. Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology: 4-Volume Set. 11<sup>th</sup> ed. Elsevier; 2016. (Accessed on July 20, 2024) URL: https://hsrc.himmelfarb.gwu.edu/books/69
3. Lamm DL. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31 Suppl 3:S86-90. DOI: 10.1086/314064
4. Hannouneh ZA, Hijazi A, Alsaleem AA, Hami S, Kheyrbek N, Tanous F, Khaddour K, Abbas A, Alshehabi Z. Novel immunotherapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med. 2023 Dec;12(24):21944-21968. DOI: 10.1002/cam4.6768
5. Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. 2006;175(6):2004-2010. DOI: 10.1016/S0022-5347(06)00264-3
6. Lamm DL, Stogdill VD, Stogdill BJ, Crispen RG. Complications of bacillus Calmette-Guerin immunotherapy in 1,278 patients with bladder cancer. J Urol. 1986;135(2):272-274. DOI: 10.1016/s0022-5347(17)45606-0
7. Grabe-Heyne K, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. Intermediate and high-risk non-muscle-invasive bladder cancer : an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023;13:1170124. DOI: 10.3389/fonc.2023.1170124
8. Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Turkeri L, Marreaud S, Collette S, Oosterlinck W. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65(1):69-76. DOI: 10.1016/j.eururo.2013.07.021
About the Authors
G. ShubhankarIndia
Gautam Shubhankar, MBBS, MS, MCH Pursuing Senior Resident
Uttarakhand; Rishikesh
Competing Interests:
The authors declare no conflicts of interest
A. Mittal
India
Ankur Mittal, MBBS, MS, MCH, Add. Prof.
Uttarakhand; Rishikesh
Competing Interests:
The authors declare no conflicts of interest
V. K. Panwar
India
Vikas K. Panwar, MBBS, MS, MCH, Ass. Prof.
Uttarakhand; Rishikesh
Competing Interests:
The authors declare no conflicts of interest
R. P. Ghorai
India
Rudra P. Ghorai, MBBS, MS, MCH, Assist. Prof.
Puducherry
Competing Interests:
The authors declare no conflicts of interest
Review
For citations:
Shubhankar G., Mittal A., Panwar V.K., Ghorai R.P. Optimizing compliance and efficacy in intravesical BCG therapy for non-muscle invasive bladder cancer: tailored strategies and clinical outcomes. Urology Herald. 2024;12(6):76-82. https://doi.org/10.21886/2308-6424-2024-12-6-76-82













































