Scroll to:
Androgen receptor gene CAG-trinucleotide repeat length affects function of endothelium in men with hypogonadism and type 2 diabetes mellitus
https://doi.org/10.21886/2308-6424-2024-12-4-14-22
Abstract
Introduction. The influence of the length of the number of CAG repeats in the androgen receptor gene (nCAG AR) on endothelial dysfunction (EnD) is currently understudied.
Objective. The study aimed to evaluate the relationship between the nCAG AR and the dynamics of biochemical and ultrasound markers of EnD in men with functional hypogonadism and type 2 diabetes mellitus (T2DM) receiving testosterone replacement therapy (TRT).
Materials & methods. This study included 45 hypogonadal men with T2DM, receiving TRT for 1 year. Patients were assessed for carbohydrate and lipid metabolism parameters; total and free T; sex hormone-binding globulin; biochemical markers of EnD (NO, eNOS3, endothelin) and the nCAG AR; brachial artery (BA) vasoreactivity. Patients were divided into 3 groups: group I — 9 men with nCAG AR < 19; group II — 27 men with nCAG AR > 19 – 24; and group III — 9 men with nCAG AR >24.
Results. Patients with nCAG AR < 19 exhibited a 2-fold greater and faster increase in BA vasoreactivity on TRT compared to patients with nCAG AR 19-24 and 3-fold greater than men with nCAG AR >24 (p < 0.05). Patients with nCAG AR < 19 also demonstrated the most pronounced rise in NO and eNOS3 on TRT compared to men with nCAG AR > 24. Patients with nCAG AR < 19 experienced the most pronounced decreases in weight, waist circumference, and HbA1c on TRT compared to other patients (p < 0.05).
Conclusion. The nCAG AR length significantly affects the response to TRT in men with hypogonadism and T2DM. The most significant improvements are seen in patients with short nCAG AR.
Keywords
For citations:
Khripun I.A., Ismailov R.S., Belousov I.I., Ibishev Kh.S., Kogan M.I. Androgen receptor gene CAG-trinucleotide repeat length affects function of endothelium in men with hypogonadism and type 2 diabetes mellitus. Urology Herald. 2024;12(4):14-22. https://doi.org/10.21886/2308-6424-2024-12-4-14-22
Introduction
The Framingham Study showed more than four decades ago that under age 60 men have more than twice the risk of developing cardiovascular disease than women [1]. For a long time, this pattern was associated with a supposed positive effect of estrogen and a suspected negative influence of testosterone (T) on the cardiovascular system. However, it has been shown that in men 35-40 years old, an annual decrease in total and free T levels occurs by 1 – 2% [2].
Therefore, 38.7% of men over 40 years of age are T deficient [3], which can lead to a reconsideration of the concept of the negative effect of T on the cardiovascular system. Indeed, the presence of endogenous T deficiency in men is related to a higher risk of mortality than in high-androgen men [4][5]. Reduced T levels lead not only to sexual and reproductive dysfunction but also to several metabolic disorders [6]. Low T levels have been shown to have a negative impact on numerous factors associated with cardiovascular risk, such as insulin resistance (IR), type 2 diabetes mellitus (T2DM), dyslipidemia and visceral obesity [7]. The presence of each of these factors contributes to endothelial dysfunction (EnD), and their combination synergistically exacerbates the pathological process [7]. EnD is considered the universal trigger for any vascular pathology and a marker of progression [8]. The earliest signs of EnD are endothelial secretory dysfunctions that occur before the deterioration of vasomotor functions and clinical manifestations of cardiovascular diseases [9].
In patients with T2DM, diabetes-induced EnD causes macrovascular complications characterized by decreased nitric oxide (NO) bioavailability, increased synthesis of prostacyclin and vasoconstrictors, endothelium-dependent hyperpolarization, and microvascular ones marked by decreased NO release, increased oxidative stress, increased production of inflammatory factors, impaired angiogenesis, and endothelial repair [10].
Endothelial NO synthesis is mediated by the endothelial NO synthase type 3 (eNOS3) enzyme. Endothelial well-being depends on the activity of eNOS3 and the rate and amount of NO synthesized [11]. An increase in vasoconstrictor substances, such as endothelin-1, reflects the functional tension of the endothelium and characterizes diseases of the cardiovascular system [12].
It was found that T has antiproliferative and vasorelaxing effects on vascular smooth muscle cells, which explains its antiatherogenic and vasoprotective effects [13]. It is assumed that the main vasodilating mechanism of androgens is the enhancement of the production and release of endothelial NO [14]. Impaired endothelium-dependent vasodilation due to a decrease in NO release in patients with hypogonadism serves as evidence of this mechanism. This process can be reversed by testosterone replacement therapy (TRT) [15].
One of the mechanisms through which T may be involved is through the CAG repeat polymorphism within the androgen receptor (AR) gene, which controls androgen sensitivity. The AR gene is located on the X chromosome. Exon 1 of the AR gene contains a sequence of CAG repeats, which encodes the transcriptional activity of AR [16]. The CAG triplet encodes the amino acid glutamine, the amount of which determines the strength of the T-receptor bond. A short number of CAG trinucleotide repeats in the AR receptor gene (nCAG AR) indicates greater sensitivity to androgens due to a stronger T-receptor bond. In contrast, long nCAG AR triplets are associated with low sensitivity to androgens due to the weak bond between the steroid hormone and the receptor [17].
The relationships between the nCAG AR and prostate cancer incidence and male fertility have been established [18][19]. The genomic mechanisms of androgen action are universal and affect extragonadal targets. The nCAG AR gene is positively correlated with the content of adipose tissue, as well as its secretory activity [20].
Genomic mechanisms of T action on the cardiovascular system and endothelium are minimally studied. Some studies have shown an association between short nCAG AR and impaired arterial vasoreactivity in healthy young volunteers, regardless of endogenous T values [21]. Decreased AR sensitivity, determined by nCAG AR, in T2DM men seems to be accompanied by impaired endothelium-dependent vasodilation, as well as increased synthesis of EnD markers [22].
Of particular interest are the limited data on the relationship between sensitivity to androgens and the effectiveness of testosterone replacement therapy (TRT) in patients with functional hypogonadism. The TIMES 2 study showed that high sensitivity to androgens is positively associated with more significant dynamics of insulinemia, triglyceridemia and IR against the background of TRT, while there was no effect on the level of HbA1c or other carbohydrate and lipid profile parameters [23].
Summarizing the analyzed data, it can be argued that the endothelium is an important target for the action of sex hormones, and EnD is one of the key factors of cardiovascular risk in men. However, studies of the genetic aspects of T action on the endothelium in men with hypogonadism receiving TRT have not been performed.
Thus, this study aimed to evaluate the relationship between the nCAG AR and the dynamics of biochemical and ultrasound markers of EnD in men with functional hypogonadism and T2DM receiving TRT.
Materials and methods
Ethics statement. The study design and protocol were reviewed and approved by the Ethics Committee of the Rostov State Medical University (Protocol No. 13/14 dated 11-09-2014). All patients gave informed written consent according to protocol also ratified by the Ethics Committee of the Rostov State Medical University.
Patient demographics and sampling. This study included 45 men with T2DM according to the criteria of the American Diabetes Association (2021). The context of this study refers to biological male sex. Moreover, men were diagnosed with potential functional hypogonadism according to the diagnostic criteria of the EAU Guidelines on Male Hypogonadism (2021). The exclusion criteria were primary or secondary hypogonadism of any origin (testicular damage, diagnosis of pituitary/hypothalamic malfunction), hyperprolactinemia and hypothyroidism, any history of a malignant disease, elevated PSA (> 4 ng/mL) and a desire for paternity.
Diagnostic work-up. Patients underwent the following anthropometric measurements: height, body weight, body mass index (BMI), waist circumference (WC), blood pressure and heart rate. The laboratory tests included carbohydrate metabolism parameters (fasting blood glucose, glycosylated hemoglobin (HbA1c), immunoreactive insulin (IRI), and calculation of HOMA1-IR). Enzyme-linked immunosorbent assays (ELISA) included assessment of sex hormones (serum total T and sex hormone-binding globulin with calculation of free T according to Vermeulen, luteinizing and follicle-stimulating hormones) and biochemical markers of EnD.
Blood was collected from the ulnar vein before 10 am after 12 h of fasting. Biochemical tests were performed in fresh blood serum; for enzyme immunoassays, blood was centrifuged at 3000 rpm/20 min and serum was frozen at -20 °C for analysis of all parameters in a batch.
The Bayer ADVIA 1650 analyser (EKF Diagnostics USA, Stabio Labs, USA) was used to assess biochemical markers. HbA1c was measured on the DCA 2000+ analyser (Siemens Healthineers AG, Germany). To determine IR, the HOMA1-IR index was calculated using the homoeostasis model as follows: (HOMA1-IR: fasting insulin (µU/ml) × fasting glucose (mmol/l)/22.5).
Total T, sex hormone binding globulin, luteinizing and follicle-stimulating hormones were determined using the DRG ELISAs laboratory kits (DRG Diagnostics GmbH, Germany). To assess the serum IRI concentrations, laboratory kits (Monobind, Inc., USA) were used. Serum concentrations of EnD markers were assessed by laboratory kits for ELISA: NO, (R&D, USA); eNOS3, (BCM Diagnostics, USA); endothelin-1, (Biomedica Medicin Product GmbH, Austria); C-reactive protein, (Biomerica Inc., USA); ICAM-1, VCAM-1, VEGF-1, p- и е-selectins (Bender Medsystems GmbH, Germany). The presence of CAG repeats polymorphism in the AR gene was determined in all patients using a PCR with analysis of polymorphism of amplification fragment lengths. The study material was genomic DNA isolated from blood leukocytes using the Wizard ® Genomic DNA Purification Kit (Promega Corp., USA).
The brachial artery (BA) vasoreactivity was assessed following the method of D. Celermejer. A cuff was stretched in the brachial upper third, the pressure was pumped 40 mmHg higher than the systolic blood pressure in the BA. Compression was maintained for 5 min. Afterwards, the cuff pressure was sharply reduced, resulting in rapid decompression with increased blood flow and change in BA diameter. The diameter of the BA was measured 10 times using the HD 11 XE ultrasound system (Koninklijke Philips Electronics N.V., the Netherlands) [ 7.0 MHz linear sensor] — at 15 sec, 30 sec, 1 min, 1 min 30 sec, 2 min, 3 min, 4 min, 5 min, and 10 min after decompression [24]. A complete reassessment of the baseline parameters was performed at the end of the study after 1 year.
Groups of patients. Patients were divided into 3 groups according to the nCAG AR detected. Group I included 9 men (mean age 53.3 ± 5.4 years) with short nCAG AR < 19. Group II included 27 men (mean age 54.1 ± 5.6 years) with medium nCAG AR 19 – 24. Group III included 9 men (mean age 53.7 ± 5.2 years) with long nCAG AR > 24.
Patient therapy. The efficacy of antidiabetic therapy was comparable among the groups and did not change during the entire study period. These agents included metformin, sulfonylurea, and DPP-4 inhibitors. All patients received T-Gel at a dose of 50 mg q.d. TD was applied to the skin in the morning daily (serum sampling was performed 3 – 4 hours after application of T-Gel). None of the initial 45 patients discontinued the study.
Statistics. Statistical analysis was carried out using the STATISTICA ver. 10.1 (StatSoft Inc., USA) software package. All variables were checked for normality of distribution according to the Kolmogorov‒Smirnov–Lilliefors method. Since most of the data were not normally distributed, they are presented as medians [lower quartile; upper quartile]. Fewer normally distributed data are presented as the mean (M) ± standard deviation (SD). Statistical analysis was carried out using the Wilcoxon signed rank test and the Kruskal‒Wallis test. The accepted significance level of the differences was p < 0.05.
Results
During the treatment period, no serious adverse events were registered, PSA levels remained < 4 ng/mL for all subjects, and hematocrit levels remained < 52% for all subjects. The baseline and follow-up parameters are presented in table.
Table. Changes in anthropometric, hormonal, metabolic variables,
and levels of endothelial dysfunction markers in men with hypogonadism
and type 2 diabetes mellitus receiving testosterone replacement therapy
|
Variables |
Baseline |
Follow – up (1 year) |
P* |
|
Anthropometric data |
|||
|
Body mass (kg) |
103 [ 97; 117] |
98.2 [ 91.2; 105] |
0.001 |
|
BMIa (kg/m²) |
32.8 [ 30.6; 36.9]; |
30.0 [ 27.8; 34.2] |
0.001 |
|
WCb (cm) |
112 [ 106; 125] |
107 [ 103; 116] |
0.046 |
|
HCc (cm) |
110 [ 104; 117] |
107 [ 101; 113] |
0.087 |
|
Sex hormones |
|||
|
Total testosterone (nmol/l) |
9.9 [ 6.8; 11.2] |
14.6 [ 13.5;17.4] |
< 0.001 |
|
SHBGd (nmol/l) |
22.2 [ 11.8; 32.4] |
23.1 [ 14.7; 31.2] |
0.941 |
|
Free testosterone (pmol/ml) |
222 [ 150; 237] |
319.5 [ 291; 444] |
< 0.001 |
|
Carbohydrate metabolism |
|||
|
Fasting glucose (mmol/l) |
7.17 [ 6.3; 9.1] |
6.2 [ 5.8; 7.4] |
0.003 |
|
IRIe (mcME/ml) |
20.0 [ 10.7; 32.4] |
12.2 [ 7.3; 25.8] |
0.038 |
|
НОМА1-IRf (U) |
8.1 [ 4.2; 10.6] |
4.1 [ 2.1; 7.8] |
0.003 |
|
HbA1c (%) |
7.2 [ 6.4; 8.7] |
6.6 [ 6.1; 7.4] |
0.01 |
|
Markers of endothelial dysfunction |
|||
|
NO (mkmol/l) |
79.8 [ 70.4; 91.2] |
183.4 [ 132.1;237.8] |
< 0.001 |
|
eNOS3g (pg/ml) |
143.3 [ 110.6;234.6] |
274.8 [ 195.2;421.3] |
< 0.001 |
|
Endothelin (fmol/ml) |
1.5 [ 1.1;1.9] |
0.6 [ 0.4; 1.1] |
0.001 |
|
VCAM-1h (ng/ml) |
937 [ 826; 1035] |
805.3 [ 523;1093] |
0.167 |
|
ICAM-1i (ng/ml) |
338.7 [ 269.5; 419] |
209.6 [ 170; 260.4] |
< 0.001 |
|
p-selectin (ng/ml) |
603.2 [ 537.2;652.5] |
129.9 [ 89.7; 163] |
< 0.001 |
|
e-selectin (ng/ml) |
43.0 [ 32.0;69.6] |
33.0 [ 22; 61.5] |
0.091 |
|
Cadherin (ng/ml) |
1.3 [ 1.1; 2.5] |
0.5 [ 0.4; 4.0] |
0.01 |
|
С-reactive protein (mg/l) |
10.1 [ 5.9; 13.9] |
2.8 [ 1.0; 10.5] |
0.001 |
Note. The values are presented as medians [lower quartile;
upper quartile]. All male patients (n = 45) were selected for analysis.
* Baseline vs follow-up values (p < 0.05), W signed rank test
a BMI — body mass index; b WC — waist circumference; c HC — hip circumference;
d SHBG — sex hormone-binding globulin; e IRI — immunoreactive insulin;
f НОМА1-IR — homeostatic model assessment-1 – insulin resistance;
geNOS3 — endothelial nitric oxide synthase 3; h VCAM-1 — vascular cell adhesion molecule 1;
i ICAM-1 — intercellular adhesion molecule 1
Changes in metabolism. TRT in patients with hypogonadism and T2DM resulted in a reduction in body mass (p = 0.001), BMI (p = 0.001), and WC (p = 0.046); improved carbohydrate metabolism parameters, such as fasting glucose (p = 0.003), IRI (p = 0.038), HOMA1-IR (p = 0.003) and HbA1c (p = 0.01); and an increase in the concentration of total and free T (p < 0.001). Moreover, T-cell therapy in men with hypogonadism and T2DM was accompanied by an improvement in endothelial function, characterized by an increase in NO and eNOS3 levels (p < 0.001) and a decrease in the level of endothelin (p = 0.001). However, the magnitude of the response to TRT was contingent on the nCAG AR.
It is important to note that the increase in the serum T concentration following TRT did not depend on the nCAG AR. Thus, the delta of total T in group I was 7.2 [ 2.9; 11.6] nmol/L, that in group II was 5.9 [ 3.2; 8.9] nmol/L, and that in group III was 5.4 [ 2.5; 7.7] nmol/L (p = 0.862). Therefore, the differences that will be described below are due precisely to the AR sensitivity.
Changes in body weight and WC are presented in Fig. 1A, 1B. Body weight decreased by 1.4 times more in men with a short nCAG AR than in those with an average length and by 2.5 times more than in those with a long nCAG AR (p = 0.004). Moreover, it is noteworthy that patients with > 24 nCAG AR repeats had less weight loss, and as the upper quartile demonstrates, some patients even had an increase in body weight. Similar findings were obtained for WC, which decreased 5-fold in men with long nCAG AR compared with patients with short nCAG AR.
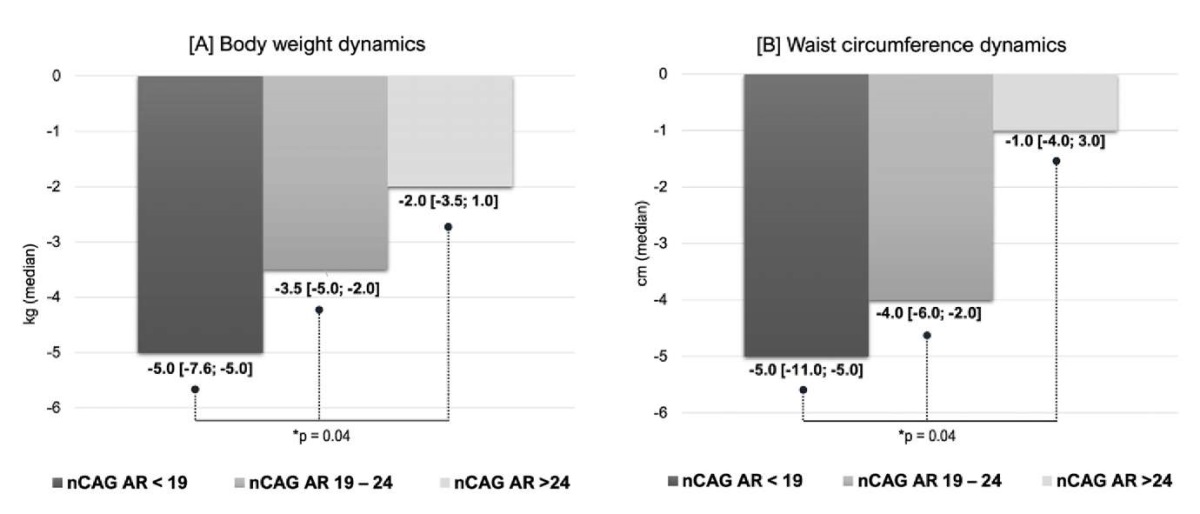
Figure 1. Influence of the nCAG repeats in the androgen receptor gene
on the dynamics of body weight [1A] and waist circumference [1B]
in men with hypogonadism and type 2 diabetes mellitus
receiving testosterone replacement therapy (*K‒W test)
The study of carbohydrate and lipid metabolism revealed statistically significant differences only in relation to HbA1c. The dynamics of HbA1c were the highest in group I (p = 0.03) and amounted to 1.2%, while in patients in group III, this parameter did not change against the baseline. Neither the fasting glycemia nor the hyperinsulinemia level was significantly different.
Changes in vasoreactivity. The length of the nCAG AR has shown a strong association with the dynamics of endothelium-dependent vasodilation of the BA and the timing of the development of maximal vasodilation during the test of reactive hyperemia. Thus, patients with a nCAG AR < 19 exhibited a two-fold increase in arterial vasoreactivity on TRT compared to individuals with a nCAG AR > 24 and a three-fold increase (p < 0.05) compared to men with an nCAG AR > 24 (Fig. 2A, 2B). Moreover, in patients with a short nCAG AR and greater androgen receptor sensitivity, vasodilation of the BA occurred twice as fast as that in other patients (p < 0.05). Interesting data have been obtained related to biochemical markers of EnD. Changes in NO and eNOS3 concentrations (Fig. 3A, 3B) were unidirectional.
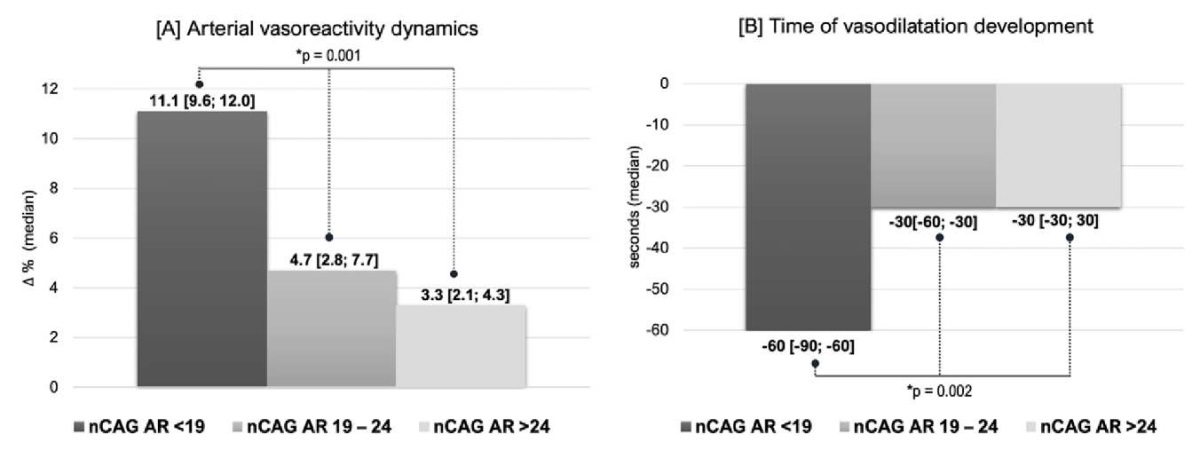
Figure 2. Influence of the nCAG repeats in the androgen receptor gene
on the dynamics of arterial vasoreactivity in the brachial artery [2A]
and the time of vasodilation [2B] in men with hypogonadism and type 2 diabetes mellitus
receiving testosterone replacement therapy (*K‒W test)
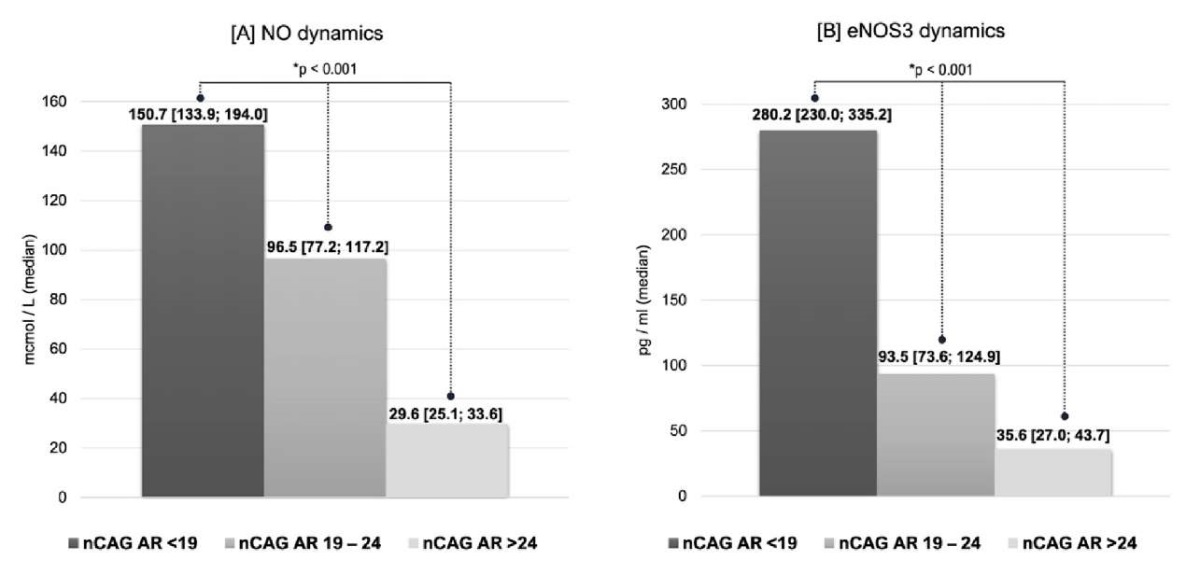
Figure 3. Influence of the nCAG repeats in the androgen receptor gene
on the dynamics of nitric oxide (NO) [3A]
and endothelial nitric oxide synthase 3 (eNOS3) [3B] levels
in men with hypogonadism and type 2 diabetes mellitus
receiving testosterone replacement therapy (*K‒W test)
Patients with a nCAG AR < 19 had the most pronounced increase in the concentration of NO in TRT, which was 1.6 times greater than that in men with a nCAG AR 19 – 24 and 5.1 times greater than that in men with a nCAG AR > 24. Even more significant was the change in eNOS3 levels, which was 3 times greater in group I than in group II and 8 times greater in group II than in group III. Changes in the endothelin concentration during T-cell therapy did not significantly correlate with AR gene polymorphisms or, consequently, with tissue receptor sensitivity to androgen therapy.
Discussion
Research by Heald A.H. et al. showed that decreased androgen sensitivity is associated with increased mortality in men with T2DM [25]. However, what are the mechanisms underlying this phenomenon? And why do hypogonadal patients treated with TRT show varying levels of effectiveness? It should be emphasized that studies on the influence of T on endothelial function are rare worldwide, and there is no research on the effects of T on the cardiovascular system.
We examined men with functional hypogonadism and T2DM who received TRT for one year and were divided into groups according to the nCAG AR. Importantly, the T concentration on TRT in all groups changed comparably, which made it possible to precisely judge the effect of the sensitivity of AR on the above parameters.
Visceral obesity and HbA1c levels during TRT decreased more markedly in men with high sensitivity to AR. No relationship was found between the nCAG AR and changes in other indicators of carbohydrate and lipid metabolism during TRT. Overall, the available data on the effects of androgen sensitivity on lipid and carbohydrate metabolism are conflicting. Thus, in contrast to our study, the TIMES2 study revealed a positive association between the nCAG AR and fasting insulin and triglyceride levels [23]. In addition, a positive correlation between the nCAG AR and insulin levels in young healthy men was found [26]. However, in a population of South Asian men, there was no association between the nCAG AR and IR [27]. A Chinese study revealed an association between hypertension and nCAG AR and high-density lipoprotein levels [28]. However, the patient cohorts in these studies and ours are not comparable. Based on the results of our study, we can determine the nonsignificant influence of androgen sensitivity on carbohydrate metabolism and the absence of an effect on lipid metabolism in men with T2DM and hypogonadism receiving TRT.
The most important information was obtained about the relationship between the nCAG AR polymorphism and vasomotor and secretory functions dynamics of the endothelium in men receiving TRT. Traditionally, the percentage change in BA diameter from baseline, i.e. arterial vasoreactivity, has been used to assess flow-mediated vasodilation. However, we paid attention to another parameter — the time at which the maximum BA vasodilation developed. Our previous study showed that increasing this parameter is one of the earliest signs of EnD and appears long before the change in arterial vasoreactivity, even in the absence of clinical symptoms of cardiovascular disease [9, 24]. Moreover, it has been shown that the slowdown in vasodilation occurs along with its weakening in men with hypogonadism and T2DM [29]. A detailed ultrasound study of endothelial function revealed that in patients receiving TRT with short nCAG AR, endothelium-dependent BA vasodilation was more pronounced and occurred faster than in men with medium and long nCAG AR. AR sensitivity synergistically determines the spatial and temporal characteristics of endothelium-dependent vasodilation.
The physiological explanation for this phenomenon lies in the modeling of secretory activity in the endothelium by AR sensitivity. The closest relationship was found between the nCAG AR and NO production. The concentrations of NO and eNOS3 during TRT increased maximally in men with the nCAG AR < 19 and minimally when the nCAG AR > 24. The dynamics in endothelin concentrations did not depend on the length of the AR gene alleles.
That is, at comparable levels of total and free T, men with a longer nCAG AR, associated with low androgen sensitivity, have worse indicators of endothelial function on TRT, weakening of the vasodilator and vasoprotective properties of NO.
Thus, one of the key conclusions is that the nCAG AR is significantly related to improvements in the vasomotor and secretory functions of the endothelium during TRT, the positive dynamics of which are most pronounced in patients with high sensitivity of receptors to androgens (nCAG AR < 19) compared to patients with moderate (nCAG AR 19 – 24) or low sensitivity (nCAG AR > 24) to androgens.
Limitations. All patients enrolled in the study were diagnosed with T2DM; however, these findings cannot be extrapolated to the population of men with T deficiency without carbohydrate metabolism disturbances, and further research is needed.
Conclusion
The nCAG AR significantly influences the response to TRT in men with functional hypogonadism and T2DM. TRT in hypogonadal men with T2DM enhances endothelial function, with the most substantial improvements observed in patients with short nCAG AR and high AR sensitivity. Conversely, a long repeat length, which modulates low androgen sensitivity, is associated with the least significant alterations in biochemical and ultrasonographic markers of EnD on TRT.
References
1. Price MA, Alvarado BE, Rosendaal NTA, Câmara SMA, Pirkle CM, Velez MP. Early and surgical menopause associated with higher Framingham Risk Scores for cardiovascular disease in the Canadian Longitudinal Study on Aging. Menopause. 2021;28(5):484-90. DOI: 10.1097/GME.0000000000001729
2. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589-98. DOI: 10.1210/jcem.87.2.8201
3. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-9. DOI: 10.1111/j.1742-1241.2006.00992.x
4. Boden WE, Miller MG, McBride R, Harvey C, Snabes MC, Schmidt J, McGovern ME, Fleg JL, Desvigne-Nickens P, Anderson T, Kashyap M, Probstfield JL.Testosterone concentrations and risk of cardiovascular events in androgen-deficient men with atherosclerotic cardiovascular disease. Am Heart J. 2020;224:65-76. DOI: 10.1016/j.ahj.2020.03.016
5. Islam RM, Bell RJ, Handelsman DJ, McNeil JJ, Nelson MR, Reid CM, Tonkin AM, Wolfe RS, Woods RL, Davis SR. Associations between blood sex steroid concentrations and risk of major adverse cardiovascular events in healthy older women in Australia: a prospective cohort substudy of the ASPREE trial. Lancet Healthy Longev. 2022;3(2):e109-e118. DOI: 10.1016/S2666-7568(22)00001-0
6. Mederos MA, Bernie AM, Scovell JM, Ramasamy R. Can Serum Testosterone Be Used as a Marker of Overall Health? Rev Urol. 2015;17(4):226-30. PMCID: PMC473566
7. Kirby M, Hackett G, Ramachandran S. Testosterone and the Heart. Eur Cardiol. 2019;14(2):103-10. DOI: 10.15420/ecr.2019.13.1
8. Veerasamy M, Bagnall A, Neely D, Allen J, Sinclair H, Kunadian V. Endothelial dysfunction and coronary artery disease: a state-of-the-art review. Cardiol Rev. 2015;23(3):119-29. DOI: 10.1097/CRD.0000000000000047
9. Khripun IA, Vorobyev SV, Morgunov MN, Kogan MI. Endothelial function in men with type 2 diabetes without clinical signs of cardiovascular disease. Diabetes mellitus. 2016;19(5):383-7. DOI: 10.14341/DM8017
10. Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434-49. DOI: 10.1111/1753-0407.12521
11. Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. 2020;36(2):307-21. DOI: 10.1016/j.ccc.2019.12.009
12. Dubsky M, Veleba J, Sojakova D, Marhefkova N, Fejfarova V, Jude EB. Endothelial dysfunction in diabetes mellitus: new insights. Int J Mol Sci. 2023;24(13):10705. DOI: 10.3390/ijms241310705
13. Lorigo M, Mariana M, Lemos MC, Cairrao E. Vascular mechanisms of testosterone: the nongenomic point of view. J Steroid Biochem Mol Biol. 2020;196:105496. DOI: 10.1016/j.jsbmb.2019.105496
14. Hotta Y, Kataoka T, Kimura K. Testosterone deficiency and endothelial dysfunction: nitric oxide, asymmetric dimethylarginine, and endothelial progenitor cells. Sex Med Rev. 2019;7(4):661-68. DOI: 10.1016/j.sxmr.2019.02.005
15. Sansone A, Rastrelli G, Cignarelli A, de Rocco Ponce M, Condorelli RA, Giannetta E, Maseroli E, Pinto S, Salzano C, Santi D. Effect of treatment with testosterone on endothelial function in hypogonadal men: a systematic review and meta-analysis. Int J Impot Res. 2020;32(4):379-86. DOI: 10.1038/s41443-019-0163-6
16. Online Mendelian Inheritance in Man® (OMIM®). OMIM® Database [Internet]. *313700 Androgen receptor (AR) gene-phenotype relationships. Baltimore (MD), the USA: McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University School of Medicine. ©1966 – 2023. [cited 2022 Oct 17]. Available from: https://www.omim.org/entry/313700.
17. Gerald T, Raj G. Testosterone and the androgen receptor. Urol Clin North Am. 2022;49(4):603-14. DOI: 10.1016/j.ucl.2022.07.004
18. Aurilio G, Cimadamore A, Mazzucchelli R, Lopez-Beltran A, Verri E, Scarpelli M, Massari F, Cheng L, Santoni M, Montironi R. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells. 2020;9(12):2653. DOI: 10.3390/cells9122653
19. , Walker WH. Androgen actions in the testis and the regulation of spermatogenesis. ADV Exp Med Biol. 2021;1288:175-203. DOI: 10.1007/978-3-030-77779-1_9
20. McEwan IJ, Brinkmann AO. Androgen physiology: receptor and metabolic disorders. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. editor(s). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2021.
21. Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasore-activity. J Clin Endocrinol Metab. 2001;86(10):4867-73. DOI: 10.1210/jcem.86.10.7889
22. Khripun IA, Vorobyev SV, Kogan MI. Association of the polymorphism in the androgen receptor gene and endothelial function in men with type 2 diabetes. Diabetes mellitus. 2015;18(4):35-40. DOI: 10.14341/DM7622
23. Stanworth RD, Akhtar S, Channer KS, Jones TH. The role of androgen receptor CAG repeats polymorphism and other factors which affect the clinical response to testosterone replacement in metabolic syndrome and type 2 diabetes: TIMES2 substudy. Eur J Endocrinol. 2013;170(2):193-200. DOI: 10.1530/EJE-13-0703
24. Khripun IA, Morgunov MN, Vorobyev SV, Terentiev VP, Kogan MI. Endothelial dysfuncton and 2 type diabetes: novel markers for earlier diagnostics. Cardiovascular Therapy and Prevention. 2016;15(5):59-63. DOI: 10.15829/1728-8800-2016-5-59-63
25. Heald AH, Yadegar Far G, Livingston M, Fachim H, Lunt M, Narayanan RP, Siddals K, Moreno G, Jones R, Malipatil N, Rutter M, Gibson M, Donn R, Hackett G, Jones H. Androgen receptor-reduced sensitivity is associated with increased mortality and poorer glycemia in men with type 2 diabetes mellitus: a prospective cohort study. Cardiovasc Endocrinol Metab. 2020;10(1):37-44. DOI: 10.1097/XCE.0000000000000230
26. Möhlig M, Arafat AM, Osterhoff MA, Isken F, Weickert MO, Spranger J, Pfeiffer AF, Schöfl, C. Androgen receptor CAG repeat length polymorphism modifies the impact of testosterone on insulin sensitivity in men. European journal of endocrinology. 2011;164(6):1013-8. DOI: 10.1530/EJE-10-1022
27. Malavige LS, Jayawickrama S, Ranasinghe P, Levy JC. Androgen receptor CAG repeat polymorphism is not associated with insulin resistance and diabetes among South Asian males. BMC Res Notes. 2017;10(1):685. DOI: 10.1186/s13104-017-3035-5
28. Yang D, Tian J, Zhang X, Yu J, Li S, Wang Z, Ma Y, Liu L, Huang Q, Ma R, Wang J, Li X, Jiang M. The polymorphic CAG repeats in exon 1 of androgen receptor is associated with level of HDL cholesterol and hypertension in Chinese middle-aged and elderly men. Clin Endocrinol (Oxf). 2017;87(1):29-34. DOI: 10.1111/cen.13326
29. Khripun IА, Vorobyev SV. Endothelial function status in hypogonadal men. Diabetes Mellitus. 2021;24(5):440-7. DOI: 10.14341/DM12780
About the Authors
I. A. KhripunRussian Federation
Irina A. Khripun — Dr.Sc.(Med), Assoc. Prof. (Docent).
Rostov-on-Don
Competing Interests:
None
R. S. Ismailov
Russian Federation
Ruslan S. Ismailov — Cand.Sc.(Med).
Rostov-on-Don
Competing Interests:
None
I. I. Belousov
Russian Federation
Igor I. Belousov — Dr.Sc.(Med), Assoc.Prof. (Docent).
Rostov-on-Don
Competing Interests:
None
Kh. S. Ibishev
Russian Federation
Khalid S. Ibishev — Dr.Sc.(Med), Full Prof.
Rostov-on-Don
Competing Interests:
None
M. I. Kogan
Russian Federation
Mikhail I. Kogan — Dr.Sc.(Med), Full Prof., Hons.Sci. of the Russian Federation.
Rostov-on-Don
Competing Interests:
None
Review
For citations:
Khripun I.A., Ismailov R.S., Belousov I.I., Ibishev Kh.S., Kogan M.I. Androgen receptor gene CAG-trinucleotide repeat length affects function of endothelium in men with hypogonadism and type 2 diabetes mellitus. Urology Herald. 2024;12(4):14-22. https://doi.org/10.21886/2308-6424-2024-12-4-14-22













































