Scroll to:
The risk of developing autonomic dysreflexia during urodynamic testing in patients after spinal cord injury
https://doi.org/10.21886/2308-6424-2022-10-4-43-53
Abstract
Introduction. Autonomic dysreflexia (AD) is a life-threatening dangerous condition in patients with spinal cord injury (SCI) above the T6 segment level. It is characterized by a sudden rise in systolic blood pressure more than 20 mmHg, and unpredictable reactions from the autonomic nervous system. An episode of autonomic dysreflexia can lead to several cardiovascular catastrophes – heart attack and/or acute cerebrovascular accident up to a lethal outcome. Currently, there is no diagnostic algorithm and no way to determine risk factors for the occurrence of autonomic dysreflexia.
Objective. To search for the most informative diagnostic criteria for autonomic dysreflexia in patients with spinal cord injury.
Materials and methods. The study included 40 patients with SCI above the T6 segment. Depending on the SCI degree, two groups were distinguished. Group 1 (n = 14) included patients with complete spinal cord injury, advising category A on the ASIA scale. Group 2 (n = 26) included patients with incomplete spinal cord injury, classified as ASIA-B, C, D. For a preliminary assessment of the risk factors for the development of AD, an ADFSCI questionnaire was used, then a urodynamic study was conducted with simultaneous registration of systolic / diastolic blood pressure (SBP / DBP), and heart rate, which confirmed or denied the presence of AD in patients.
Results. According to the ADFSCI questionnaire, most patients showed a high degree of severity of autonomic disorders, suggesting the presence of AD. Subsequently, this assumption was confirmed by the results of a urodynamic testing with simultaneous monitoring of SBP / DBP and heart rate. When comparing the groups, statistically significant differences were found in the parameters of the ADFSCI questionnaire, as well as in the indicators of SBP / DBP (at the points of maximum detrusor pressure and when the cystometric capacity is reached).
Conclusion. The obtained results allow us to draw conclusions about the high incidence of AD in patients with SCI above the T6 segment and the need for a preliminary assessment of the risks of developing this condition based on the ADFSCI questionnaire before conducting a urodynamic study. Considering the possible complications of AD, the urodynamic testing should be accompanied by continuous monitoring of the indicators of the cardiovascular system.
For citations:
Kamalov A.A., Okhobotov D.A., Chaly M.E., Frolova M.V., Khutoroi I.V., Salyukov R.V. The risk of developing autonomic dysreflexia during urodynamic testing in patients after spinal cord injury. Urology Herald. 2022;10(4):43-53. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-43-53
Introduction
Between 250,000 and 500,000 people worldwide experience spinal cord injury (SCI) each year [1]. These are mostly young men aged 20 to 35 years old [2]. In Russia, according to various publications, there has been an increase in the number of patients with SCI, and currently, SCI account for 17% of all musculoskeletal injuries [3].
Among the priorities in recovery and rehabilitation after SCI, in addition to the musculoskeletal system, patients note the importance of lower urinary tract and cardiovascular system functions due to their significant impact on quality of life [4–6]. In addition, complications of the urinary tract and cardiovascular system are the leading causes of mortality in patients with SCI [7–10]. Autonomic dysreflexia (AD) is a dangerous condition that develops after SCI at or above the T6 segment and affects the cardiovascular system. It is clinically manifested as a sharp and uncontrolled increase in blood pressure (BP) up to 300 mmHg. This condition is associated with high risk of cardiovascular and cerebrovascular events and a lethal outcome [11]. Over 90% of patients with high SCI experience symptoms of AD [12][13]. According to Hubli et al. (2015), on average, a patient with SCI experiences about 11 episodes of AD during a day [14]. AD is manifested by a powerful generalized sympathetic response (blood pressure elevation, release of norepinephrine and dopamine, massive vasoconstriction) to visceral or somatic stimuli below the level of trauma [15]. The most common triggers of AD include significant stretching of the bladder wall and rectal ampulla [12][16][17], as well as urinary tract infection, bladder catheterization, invasive manipulations on the urinary tract, including urodynamic examination, cystoscopy, bladder stones, erection, and ejaculation [18][19].
Diagnostic criteria for AD are rather limited according to the guidelines of the European Association of Urologists, including only the presence of episodic hypertension (elevation of BP by over 20 mmHg) [20]. To expand the diagnostic capabilities of AD, as well as to target complaints, the authors used a preliminary questionnaire from patients followed by a urodynamic study with continuous monitoring of blood pressure and heart rate (HR).
The study aimed to search for the most informative diagnostic criteria for autonomic dysreflexia in patients with spinal cord injury.
Materials and methods
The study was conducted in the clinical facilities of the Medical Research and Education Centre and “Preodolenie” Rehabilitation Centre, Lomonosov Moscow State University from 2019 to 2022. The study included 40 patients with SCI above the T6 segment (4 women and 36 men). The mean duration of injury was 7 [ 3; 13] years, and the mean age of patients was 33.5 [ 28.75; 40.00] years old.
The patients were divided into two groups according to the severity of SCI and neurological complications:
- Group 1 – patients belonging to ASIA domain A (n = 14);
- Group 2 – patients belonging to ASIA domains B and C (n = 26).
Inclusion and exclusion criteria. Inclusion criteria were SCI above the T6 segment and age over 18 years old. Exclusion criteria were exacerbation of urinary tract infection, trauma, skin injuries (bedsores, wounds, ingrown nails, etc.), bladder stones, vesicoureteral reflux, cardiovascular diseases, and pregnancy.
Questionnaire survey. Prior to the urodynamic study, patients completed the ADFSCI questionnaire, which included an assessment of the severity and frequency of symptoms associated with episodes of SCI. The ADFSCI questionnaire was developed using the Delphi technique by a consortium of experts experienced in treating people with SCI. The study showed that this questionnaire had good sensitivity to determine the frequency and severity of AD episodes [21]. The questionnaire asked patients to assess the frequency and severity of specific symptoms (sweating, headaches, goose bump sensation (piloerection), rapid heartbeat, and so on).
Urodynamic study. The bladder and rectum were emptied in all patients before a urodynamic study of filling cystometry, according to ISC guidelines [22]. Baseline systolic (SBP) and diastolic (DBP) blood pressure, and HR were also assessed. The urodynamic study was performed on a TRITON apparatus (“Laborie Medical Technologies, Inc.”, St. Lambert, QC, Canada) in a patient's position sitting or lying on a couch with an elevated head end. Disposable aqueous urodynamic catheters were used in the study. Filling cystometry was performed using an infusion of room-temperature saline solution at a rate of 30 mL per second with simultaneous continuous cardiovascular monitoring. Cardiovascular parameters were monitored every 2 minutes during filling cystometry on an Armed PC-9000f machine (“Shenzhen Creative Industry Co., Ltd.”, Shenzhen, GD, P.R. China). BP elevation over 20 mmHg from the baseline was interpreted as an AD episode. If such symptoms as headache, sweating, piloerection, or nausea occurred, the study was stopped, and the bladder was emptied.
Statistical analysis. Data were collected and analyzed using Microsoft Office Excel 2016 (“Microsoft Corp.”, Redmond, WA, USA) and JASP v.0.16.3 (University of Amsterdam, Amsterdam, the Netherlands). The authors tested the data for the normality of distribution using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Data of quantitative variables were presented as tables with the mean (M) and standard deviation (±SD), min – max, median (Me), and interquartile range [ Q1; Q3]. The authors used the Mann-Whitney U test to analyze the quantitative variables in independent groups, the Wilcoxon’s test for related groups, and gave an exact value of the test, as well as an exact value of type I error probability (p-value). The results were considered statistically significant at p < 0.05 within a = 0.05.
Quantitative variables were visualized using box plot and raincloud plot types. Visualization of categorical variables was performed using bar charts that indicated the exact value of patients in each category.
Results
Topographical characteristic of spinal cord injury. According to the study results, more than one spinal cord segment was affected in 22 (55%) patients. C4 to C7 segments were more frequently affected (Fig. 1).
Clinical manifestations of autonomic dysreflexia. The ratio of clinical symptoms showed that the most frequent clinical manifestations were piloerection, hyperhidrosis, increased spasticity, and headache (Fig. 2).
According to the sum of clinical manifestations of AD, group 1 was statistically significantly superior to group 2 (p = 0.028), which confirms a more severe clinical picture of dysreflexia in patients with more severe SCI (Fig. 3).
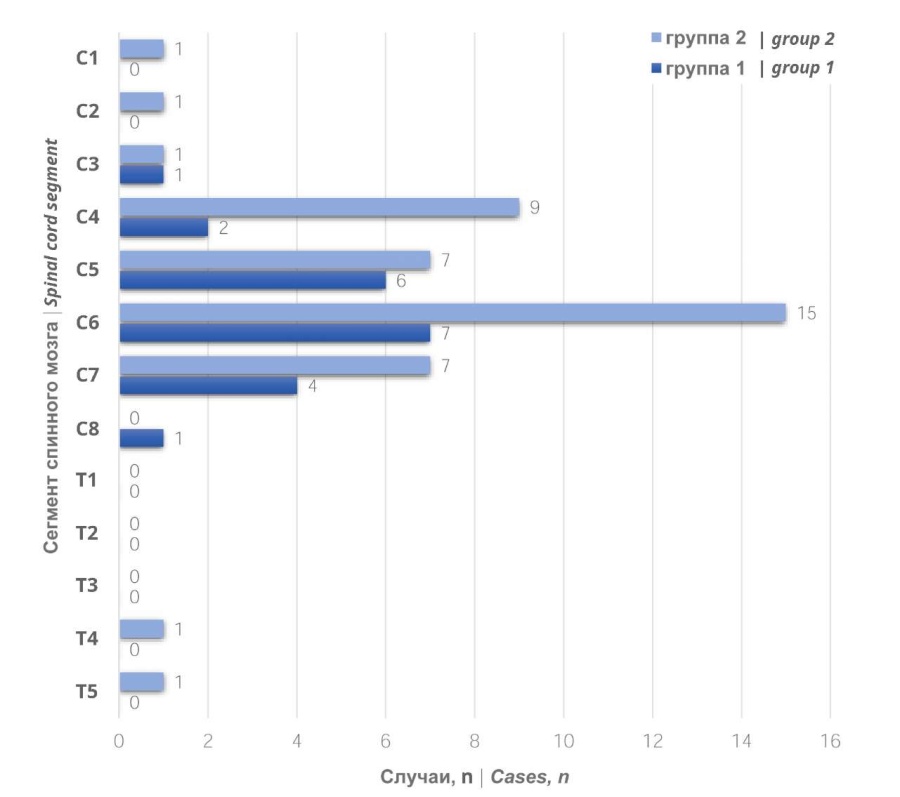
Figure 1. Quantitative distribution of cervical and upper thoracic spinal cord segment injuries (C1 — T6) in the study groups
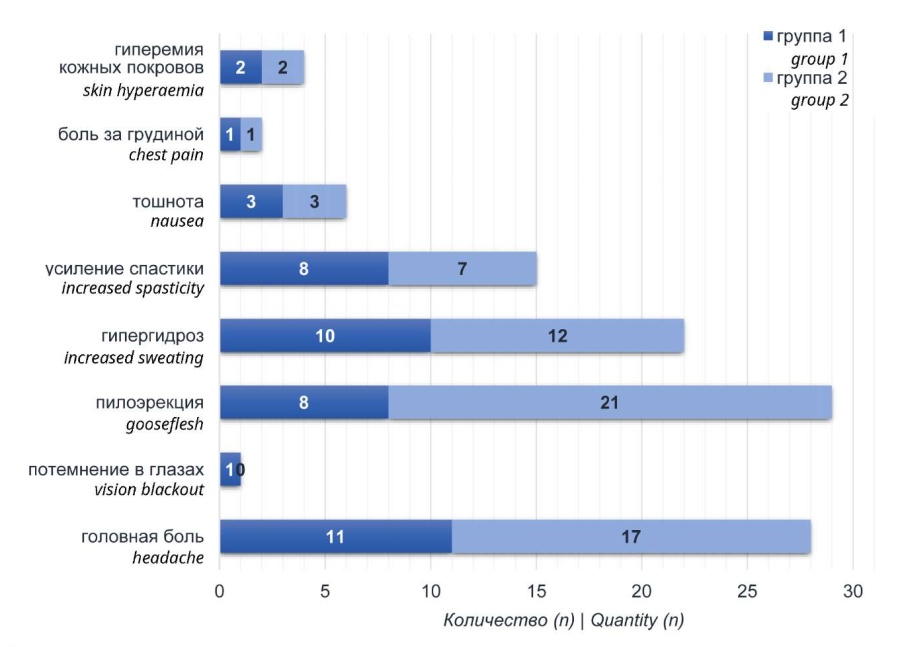
Figure 2. Clinical manifestations of autonomic dysreflexia
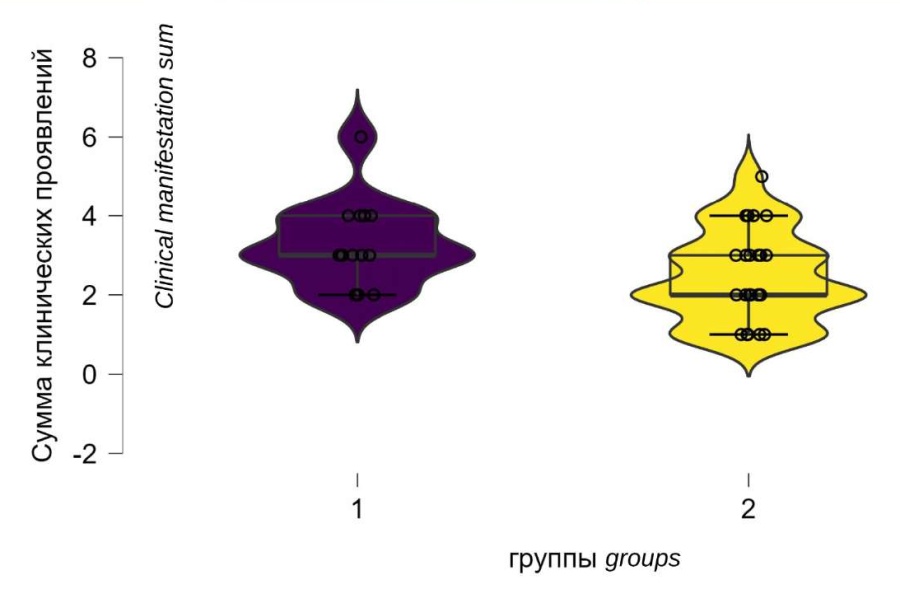
Figure 3. Clinical manifestations sum of autonomic dysreflexia
According to analysis of the dynamics of SBP, DBP and HR, there were statistically significant differences between checkpoints before urethral catheter insertion and at maximal detrusor pressure (MDP) in both groups. Thus, SBP before urethral catheter installation was 99.8 ± 17.9 mmHg, and at MDP, it was 167.35 ± 33.30 mmHg (p < 0.001). DBP before urethral catheter installation was 62.48 ± 3.55 mmHg, and at MDP, it was 103.25 ± 21.34 mmHg (p < 0.001). HR before urethral catheter installation was 63.17 ± 8.30 mmHg, and at MDP, it was 92.25 ± 27.08 mmHg (p < 0.001) (Fig. 4).
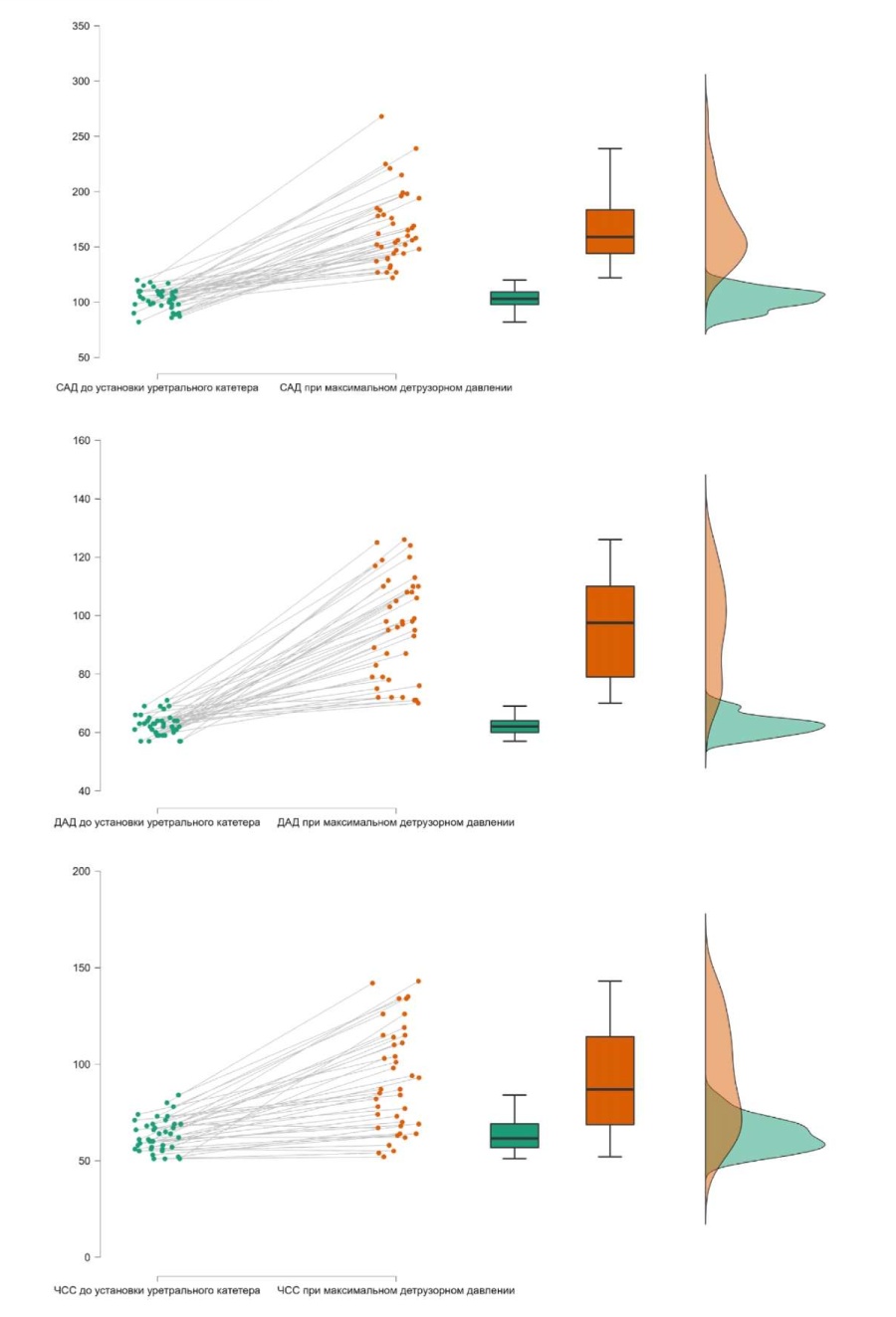
Figure 4. Dynamics of systolic/diastolic blood pressure and heart rate before the installation of a urethral catheter and upon reaching the maximum detrusor pressure
Intergroup analysis of survey questionnaire data and urodynamic study. Intergroup analysis revealed statistically significant differences in the ADFSCI questionnaire index (p = 0.002), the levels of SBP and DBP in MDP (p = 0.005 and 0.002, respectively), and the levels of SBP and DBP when reaching cystometric capacity (p = 0.005 and 0.002, respectively). At the same time, the ADFSCI questionnaire data and cardiovascular system parameters indicated a significantly less favorable picture and a more severe course of AD in patients from group 1 (ASIA – A) (Table, Fig. 5).
Table. Descriptive statistics and comparison results
|
Statistics |
Group |
p |
|||||
|
1 |
2 |
||||||
|
Q1 |
Me |
Q3 |
Q1 |
Me |
Q3 |
||
|
Age, years |
26.75 |
32.00 |
39.75 |
29.00 |
34.50 |
40.00 |
0.639 |
|
Duration of injury, years |
2.25 |
4.00 |
9.25 |
4.50 |
8.50 |
13.75 |
0.102 |
|
ADFSCI, score |
68.75 |
75.50 |
87.50 |
45.50 |
53.00 |
65.00 |
0.002 |
|
Blood pressure and heart rate before the insertion of a urethral catheter: |
|||||||
|
SBP, mmHg |
90.00 |
99.00 |
105.75 |
98.00 |
103.50 |
109.75 |
0.300 |
|
DBP, mmHg |
59.00 |
61.00 |
62.00 |
61.00 |
63.00 |
64.75 |
0.082 |
|
HR, bpm |
55.25 |
59.50 |
66.75 |
58.25 |
63.00 |
70.50 |
0.177 |
|
Blood pressure and heart rate upon reaching the cystometric capacity: |
|||||||
|
SBP, mmHg |
162.50 |
172.5 |
214.75 |
145.25 |
153.00 |
189.25 |
0.013 |
|
DBP, mmHg |
99.00 |
110.00 |
129.25 |
82.00 |
95.00 |
109.25 |
0.009 |
|
HR, bpm |
70.75 |
103.00 |
117.75 |
71.00 |
99.50 |
122.50 |
0.876 |
|
Blood pressure and heart rate at maximum detrusor pressure: |
|||||||
|
SBP, mmHg |
160.50 |
170.00 |
210.75 |
137.50 |
150.00 |
177.50 |
0.005 |
|
DBP, mmHg |
96.50 |
108.00 |
118.50 |
75.25 |
87.00 |
102.00 |
0.002 |
|
HR, bpm |
70.25 |
94.00 |
110.75 |
69.25 |
86.00 |
114.75 |
0.955 |
|
Maximum cystometric capacity, ml |
180.00 |
209.50 |
250.00 |
203.75 |
255.00 |
276.50 |
0.112 |
|
Maximum detrusor pressure, cmH20 |
34.25 |
39.50 |
49.25 |
25.25 |
31.00 |
41.00 |
0.148 |
|
Compliance, ml/cmH20 |
4.50 |
5.35 |
7.20 |
5.18 |
7.00 |
9.13 |
0.115 |
Note. ADFSCI — Autonomic Dysfunction Following Spinal Cord Injury questionnaire; SBP — systolic blood pressure; DBP — diastolic blood pressure; HR — heart rate
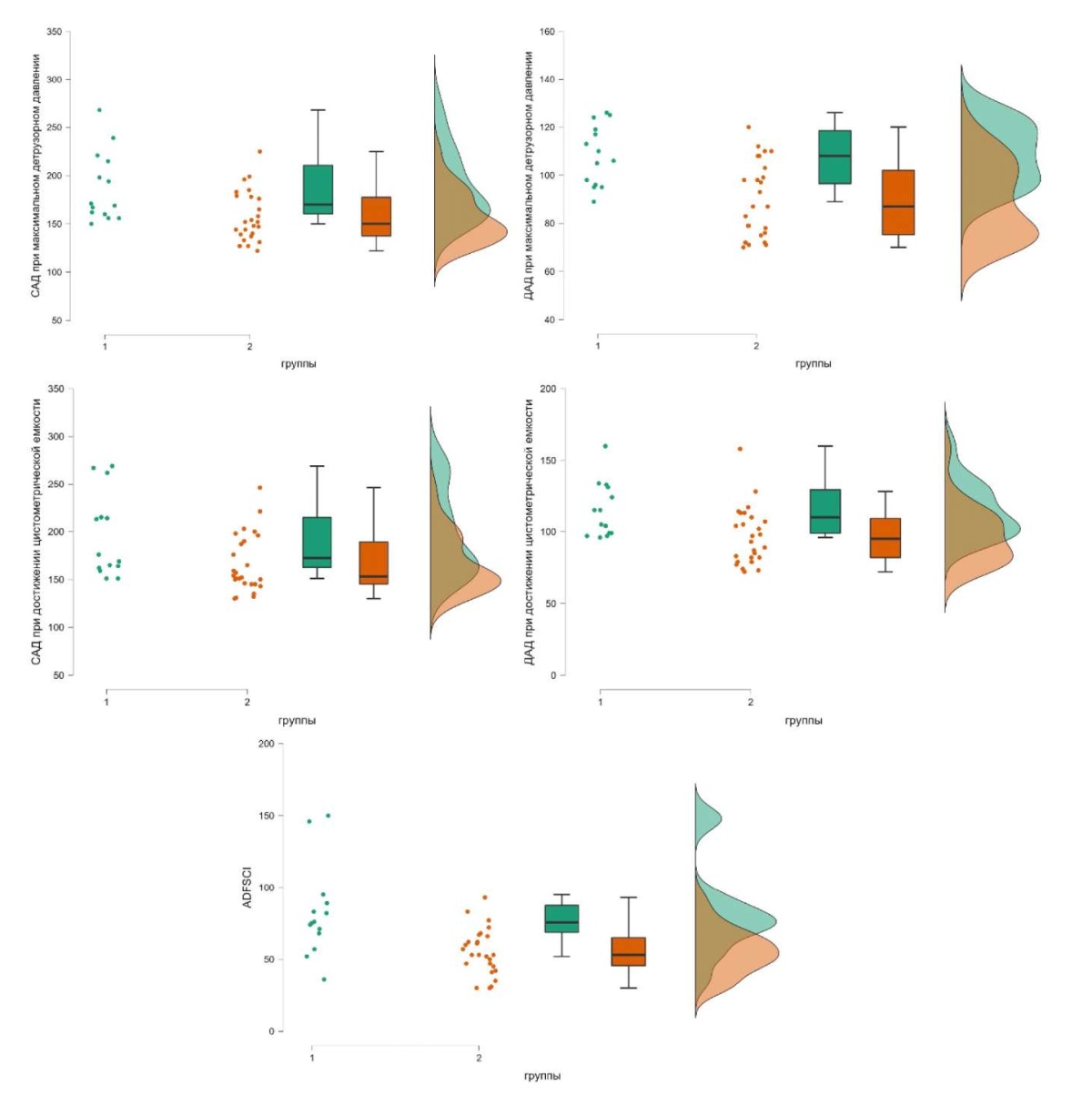
Figure 5. The differences between the study groups according to the survey scores, as well as systolic / diastolic blood pressure at checkpoints of the study
Discussion
The most frequent trigger of an episode of AD in the practice of a urologist is the performance of urological manipulations such as urodynamic examination, bladder catheterization, and cystoscopy [23][24]. The work by Faaborg et al. (2014), devoted to BP fluctuations in patients with AD, showed the figures of statistically significant elevation of SBP and DBP during a urodynamic study [25], which agrees with the results of this study. In this study, the authors compared cardiovascular parameters (SBP, DBP) in patients with complete (ASIA – A group) and incomplete SCI (ASIA – B, C) during the urodynamic study. The results of the study confirmed that when an AD episode occurred, the increase in SBP and DBP in the ASIA – A group of patients was statistically significantly higher than in the ASIA – B, C group of patients, which indicated a more severe course of AD episodes in patients with complete SCI. The clinical symptoms of AD described in the present study were consistent with all publications devoted to this topic [13][26][27]. However, clinical symptoms alone do not always reflect the severity of AD episodes and often limit the qualitative urodynamic study. The lack of a clear algorithm for diagnosing AD and methods for predicting its episodes limits the prevention and treatment of this condition. In this study, the authors showed that the patient questionnaire was a reliable way to predict the development of AD episodes during a urodynamic examination (all patients in this study had an ADFSCI score of 30 and higher). Linsenmeyer et al. (1996) investigated the response of patients with AD during the urodynamic study and emphasized that elevated BP in patients with SCI was caused by episodes of detrusor hyperactivity and bladder wall overstretching [28], which is consistent with the findings of this study (Table). A urodynamic study with continuous BP and HR monitoring is mandatory in this category of patients. Eltorai et al. (1992) described a clinical case with an episode of BP elevation to 180/90 mmHg in a patient with AD that resulted in intracranial hemorrhage and death of the patient [22]. Monitoring of vital signs during the study reflects the most complete picture of the patient's condition and allows for minimizing the risks of AD complications.
Study limitations. The main limitation of the study is the relatively small number of patients. However, since the general population of patients meeting the inclusion and non-inclusion criteria is not large, the authors believe that the number of respondents that were selected for the study was sufficient to test the research hypothesis.
Conclusion
AD is a life-threatening condition that is often encountered in patients with SCI. A more severe clinical course of AD is observed in patients with complete SCI, as demonstrated in a study in a group of patients with neurological complications (ASIA – A). A common cause of AD is invasive manipulations on the urinary tract, including complex urodynamic examination. Urodynamic study is an important and basic method of diagnosis for urinary disorders in neurogenic dysfunctions of the lower urinary tract.
Keypoints. Given the high incidence of AD during urological manipulations, a preliminary survey of patients using the ADFSCI questionnaire and continuous monitoring of cardiovascular parameters during urodynamic study allow the specialists to predict the development of AD with sufficiently high accuracy and to minimize the risk of possible complications of this condition.
References
1. Bickenbach J, Officer A, Shakespeare T, von Groote P, eds. International Perspectives on Spinal Cord Injury. World Health Organization; 2013. ISBN: 978-92-4-156466-3. URL: https://apps.who.int/iris/handle/10665/94190
2. Barbiellini Amidei C, Salmaso L, Bellio S, Saia M. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60(9):812-819. https://doi.org/10.1038/s41393-022-00795-w
3. Novoselova I.N. Etiology and clinical epidemiology of spinal cord injury. Literature review. Russian neurosurgical journal named after professor A.L. Polenov. 2019;11(4):84-92. (In. Russ) EDN: MGTZJD
4. Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol. 2015;14(7):720-32. https://doi.org/10.1016/S1474-4422(15)00070-8
5. Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus. 2008;25(5):E13. https://doi.org/10.3171/FOC.2008.25.11.E13
6. Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37(6):383-91. https://doi.org/10.1038/sj.sc.3100867
7. Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GB, Borisoff JF. Spinal cord injury and type 2 diabetes: results from a population health survey. Neurology. 2013;81(21):1864-8. https://doi.org/10.1212/01.wnl.0000436074.98534.6e
8. Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408-16. https://doi.org/10.1038/sj.sc.3101729
9. Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142-52. https://doi.org/10.1097/PHM.0b013e31802f0247
10. Sabre L, Rekand T, Asser T, Kõrv J. Mortality and causes of death after traumatic spinal cord injury in Estonia. J Spinal Cord Med. 2013;36(6):687-94. https://doi.org/10.1179/2045772313Y.0000000120
11. Wan D, Krassioukov AV. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med. 2014;37(1):2-10. https://doi.org/10.1179/2045772313Y.0000000098
12. Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18(5):285-92. https://doi.org/10.1038/sc.1980.51
13. Cragg J, Krassioukov A. Autonomic dysreflexia. CMAJ. 2012;184(1):66. https://doi.org/10.1503/cmaj.110859
14. Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens. 2015;28(2):173-81. https://doi.org/10.1093/ajh/hpu122
15. Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59-70. https://doi.org/10.1016/j.autneu.2017.05.002
16. Canon S, Shera A, Phan NM, Lapicz L, Scheidweiler T, Batchelor L, Swearingen C. Autonomic dysreflexia during urodynamics in children and adolescents with spinal cord injury or severe neurologic disease. J Pediatr Urol. 2015;11(1):32.e1-4. https://doi.org/10.1016/j.jpurol.2014.08.011
17. Snow JC, Sideropoulos HP, Kripke BJ, Freed MM, Shah NK, Schlesinger RM. Autonomic hyperreflexia during cystoscopy in patients with high spinal cord injuries. Paraplegia. 1978;15(4):327-32. https://doi.org/10.1038/sc.1977.49
18. Milligan J, Lee J, McMillan C, Klassen H. Autonomic dysreflexia: recognizing a common serious condition in patients with spinal cord injury. Can Fam Physician. 2012;58(8):831-5. PMCID: PMC3418979
19. Brown R, Burton AR, Macefield VG. Autonomic dysreflexia: Somatosympathetic and viscerosympathetic vasoconstrictor responses to innocuous and noxious sensory stimulation below lesion in human spinal cord injury. Auton Neurosci. 2018;209:71-78. https://doi.org/10.1016/j.autneu.2017.07.003
20. Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, 't Hoen L, Blok B. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol. 2016;69(2):324-33.https://doi.org/10.1016/j.eururo.2015.07.071
21. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37-49. https://doi.org/10.1016/s0090-4295(02)02243-4
22. Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Paraplegia. 1992;30(5):355-60. https://doi.org/10.1038/sc.1992.82
23. Giannantoni A, Di Stasi SM, Scivoletto G, Mollo A, Silecchia A, Fuoco U, Vespasiani G. Autonomic dysreflexia during urodynamics. Spinal Cord. 1998;36(11):756-60. https://doi.org/10.1038/sj.sc.3100684
24. Liu N, Fougere R, Zhou MW, Nigro MK, Krassioukov AV. Autonomic dysreflexia severity during urodynamics and cystoscopy in individuals with spinal cord injury. Spinal Cord. 2013;51(11):863-7. https://doi.org/10.1038/sc.2013.113
25. Faaborg PM, Christensen P, Krassioukov A, Laurberg S, Frandsen E, Krogh K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord. 2014;52(6):494-8. https://doi.org/10.1038/sc.2014.45
26. Shergill IS, Arya M, Hamid R, Khastgir J, Patel HR, Shah PJ. The importance of autonomic dysreflexia to the urologist. BJU Int. 2004;93(7):923-6. https://doi.org/10.1111/j.1464-410X.2003.04756.x
27. Allen KJ, Leslie SW. Autonomic Dysreflexia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. PMID: 29494041 Bookshelf ID: NBK482434
28. Linsenmeyer TA, Campagnolo DI, Chou IH. Silent autonomic dysreflexia during voiding in men with spinal cord injuries. J Urol. 1996;155(2):519-22. PMID: 8558650
About the Authors
A. A. KamalovRussian Federation
Armais A. Kamalov — M.D., Dr.Sc.(Med), Full Prof., Acad. of the RAS, Head, Dept. of Urology and Andrology, Faculty of Fundamental Medicine, Lomonosov Moscow State University; Headmaster, Medical Research and Educational Centre, Lomonosov Moscow State University.
1 Leninskie Gory, Moscow, 119991
Competing Interests:
The authors declare no conflicts of interest
D. A. Okhobotov
Russian Federation
Dmitry A. Ohobotov — M.D. Cand.Sc.(Med), Assoc.Prof. (Docent), Dept. of Urology and Andrology, Faculty of Fundamental Medicine, Lomonosov Moscow State University; Urologist, Medical Research and Education Center, Lomonosov Moscow State University.
1 Leninskie Gory, Moscow, 119991
Competing Interests:
The authors declare no conflicts of interest
M. E. Chaly
Russian Federation
Mikhail E. Chaliy — M.D., Dr.Sc.(Med), Full Prof., Leading Researcher, Medical Research and Education Center, Lomonosov Moscow State University.
1 Leninskie Gory, Moscow, 119991
Competing Interests:
The authors declare no conflicts of interest
M. V. Frolova
Russian Federation
Maria V. Frolova — M.D., Postgrad. Student, Dept. of Urology and Andrology, Faculty of Fundamental Medicine, Lomonosov Moscow State University.
1 Leninskie Gory, Moscow, 119991
Competing Interests:
The authors declare no conflicts of interest
I. V. Khutoroi
Russian Federation
Ivan V. Khutornoy — M.D., Postgrad. Student, Dept. of General and Advanced Surgery, Faculty of Fundamental Medicine, Lomonosov Moscow State University.
1 Leninskie Gory, Moscow, 119991
Competing Interests:
The authors declare no conflicts of interest
R. V. Salyukov
Russian Federation
Roman V. Salyukov — M.D., Cand.Sc.(Med), Assoc.Prof. (Docent), Dept. of Endoscopic Urology and Ultrasound Diagnostics, Faculty of Continuing Medical Education, Peoples’ Friendship University of Russia (RUND University).
6 Miklukho-Maklay St., Moscow, 117198
Competing Interests:
The authors declare no conflicts of interest
Review
For citations:
Kamalov A.A., Okhobotov D.A., Chaly M.E., Frolova M.V., Khutoroi I.V., Salyukov R.V. The risk of developing autonomic dysreflexia during urodynamic testing in patients after spinal cord injury. Urology Herald. 2022;10(4):43-53. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-43-53













































