Scroll to:
Treatment of post-COVID-19 patients with chronic recurrent prostatitis: efficacy of recombinant interferon α-2b medications
https://doi.org/10.21886/2308-6424-2022-10-4-32-42
Abstract
Introduction. The tactics of managing and treating patients with chronic recurrent bacterial prostatitis (CRBP) in some cases is a difficult-to-treat condition for a practicing urologist. This circumstance occurs because the disease has several predisposing factors, a complex and multifaceted pathogenesis, and certain difficulties in diagnosis and treatment.
Objective. To study the effectiveness of recombinant interferon α-2b medications in post-COVID-19 patients with chronic recurrent prostatitis against the background of antibiotic multi-drug resistance of microorganisms verified in prostate secretion.
Materials and methods. The treatment of 52 post-COVID-19 patients with CRBP was analyzed, divided into three therapy-dependent groups. Group 1 patients (n = 18) received antibiotic therapy (ABT): Levofloxacin 500 mg q.d. PO for 28 days. Group 2 patients (n = 18) underwent combined therapy: ABT supplemented with recombinant interferon α-2b with an antioxidant complex of vitamins E and C (“Viferon®” rectal suppositories) 3.000.000 IU b.i.d. PR q12h for 28 days. Group 3 patients (n = 16) received monotherapy with recombinant interferon α-2b with an antioxidant complex of vitamins E and C (“Viferon®”rectal suppositories) 3.000.000 IU b.i.d. PR q12h for 28 days. The follow-up period was 6 months with monitoring of clinical and laboratory parameters assessed before treatment, after 1, 3 and 6 months from the start of therapy.
Results. Based on the monitoring of the clinical picture and laboratory parameters, after 1 follow-up month, there was a significant decrease in the symptoms of the disease in all study groups. However, after 3 and 6 follow-up months, this trend was observed only in patients of groups 2 and 3 receiving recombinant interferon alfa-2b with an antioxidant complex (vitamins E and C).
Conclusions. Strengthening the standard CRBP-therapy with recombinant interferon α-2b with an antioxidant complex of vitamins E and C makes it possible to normalize both clinical and laboratory parameters in most patients.
For citations:
Ibishev Kh.S., Naboka Yu.L., Krainiy P.A., Gudima I.A., Plyakin A.D., Prokop J.O., Ilyash A.V. Treatment of post-COVID-19 patients with chronic recurrent prostatitis: efficacy of recombinant interferon α-2b medications. Urology Herald. 2022;10(4):32-42. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-32-42
Introduction
The approach to the treatment and management of patients with chronic recurrent bacterial prostatitis (CRBP) in some cases is a difficult task for a practicing urologist [1–3]. This circumstance is associated with the fact that the disease has some predisposing factors, complex and many-sided pathogenesis, and certain difficulties in diagnosis [4–6].
In the etiological structure of CRBP, the bacterial component is the most studied, but with a rather narrow spectrum of causative pathogens, represented mainly by Enterobacteriaceae [7][8]. However, the presence of bacteria in expressed prostatic secretion (EPS) and urine is currently considered a variant of normal because the paradigm about their sterility has been disproved [7–9].
The problem of antibiotic treatment for CRBP is in the context of the global problem of multidrug resistance exacerbated by the COVID-19 pandemic [10]. Therefore, despite the use of modern diagnostic methods and strict adherence to the examination and treatment standards of patients with this pathology in accordance with the recommendations of the European Association of Urology (EAU) and the Russian Society of Urologists (RSU), the relapse rate of the disease remains high [9][11][12]. Furthermore, the growth of microbial resistance is due to the irrational and active use of antibiotics for “mild” infectious conditions not only in urological diseases but also in entities of infectious genesis of other organs and systems [13]. Antibiotic therapy (ABT) for CRBP, especially in COVID-19 patients, is often ineffective, which dictates the need to develop and use other alternative therapies [10]. The latter particularly includes therapy with recombinant interferon α-2b [9].
The study aimed to study the effectiveness of recombinant interferon α-2b medications in post-COVID-19 patients with chronic recurrent prostatitis against the background of antibiotic multi-drug resistance of microorganisms verified in expressed prostate secretion.
Materials and methods
A prospective, randomized, comparative study was conducted that included 52 post-COVID-19 patients suffering CRBP. The uropathogens verified in their EPS had multiple antibiotic resistance to the recommended drugs.
Inclusion criteria: the patient’s age over 18 years old, verified, and registered COVID-19, clinically and laboratory confirmed diagnosis of CRBP with mild to moderate NIH-CRSI symptoms, and the patient's consent to participate in the study.
Exclusion criteria: the presence of sexually transmitted infections and other prostate diseases (hyperplasia, cancer), infectious and inflammatory diseases of the urinary tract and reproductive organs (vesiculitis, urethritis, pyelonephritis); infravesical obstructions (urethral stricture, sclerosis of the urinary bladder neck, urolithiasis, etc.), abnormalities of the urinary tract and reproductive organs, cardiovascular, neurological, endocrine, and other pathologies that could affect the study results, any immunodeficiency states, intake of antibacterial or other drugs with antibacterial, prostate-protective, anti-inflammatory, and immunostimulating effects for 30 days before inclusion in the study.
The mean age of the patients was 34.8 ± 5.2 years old; the mean duration of CRBP was 5.7 ± 2.3 years.
To assess clinical and laboratory parameters at all stages of the study, the authors analyzed the results of questionnaires using the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) and the International Prostate Symptom Score – Quality of Life questionnaire (IPSS-QoL), as well as performing laboratory and additional examination, such as native and light microscopy of EPS with Romanowsky-Giemsa staining and bacteriological examination of EPS, uroflowmetry, and transrectal ultrasound examination (TRUS).
The study design included three periods: screening, treatment, and follow-up. At the screening stage, a comprehensive clinical examination was carried out to assess the clinical and laboratory parameters of the disease before treatment. The antibiotic susceptibility of microorganisms isolated from EPS in the amount ≥ 103 CFU/mL to the recommended drugs was determined in all patients before different therapy options were prescribed [13].
During the treatment period, the patients were divided into three groups depending on the choice of therapy. Patients in group 1 (n = 18) received ABT – levofloxacin 500 mg q.d. PO for 28 days, according to the RSU guidelines. Patients in group 2 underwent combined therapy: ABT supplemented with recombinant interferon α-2b with an antioxidant complex of vitamins E and C (Viferon® rectal suppositories) 3 000 000 IU b.i.d. PR q12h for 28 days. Group 3 patients (n = 16) received monotherapy with recombinant interferon α-2b with an antioxidant complex of vitamins E and C (Viferon®, rectal suppositories), according to the above scheme.
The follow-up period was six months with the monitoring of clinical and laboratory parameters assessed before treatment, after one, three, and six months from the start of therapy. Before treatment, the groups were comparable for several parameters.
Statistical analysis. The results of the study were structured using Microsoft Office Excel 2010 (“Microsoft Corp.”, Redmond, WA, USA). Statistical data processing was performed using the Statistica ver.10.2 statistical package (“StatSoft Inc.”, Tulsa, OK, USA). The conformity of the distribution of features with the normal distribution was assessed using the Shapiro-Wilk test. Due to the lack of a normal distribution in most indicators, numerical data were represented by the median (Q2 = Me), the first and third quartiles in Me [ Q1; Q3] format. Differences in numerical scores between groups were evaluated using the Mann-Whitney U test, and the significance of differences between scores at different stages of the study compared to baseline scores was determined using the Wilcoxon test. The threshold level of significance was set at p < 0.05 with α = 0.05.
Statistical processing of the bacteriological study results was performed using the IBM SPSS Statistics 26.0 software package (“SPSS: An IBM Company”, “IBM SPSS Corp.”, Armonk, NY, USA). The detection rate (%) and 95% confidence intervals (95% CI), descriptive statistics of contamination levels (mean values (M) with 95% CI, standard deviations (± SD), median (Q2 = Me), minimum-maximum (min – max), interquartile range [ Q1; Q3]) were calculated from data from 52 patients.
Results
Various variants of aerobic-anaerobic microbial compositions were registered in EPS of all patients. A total of 27 microbiota taxa were identified. The aerobic cluster was represented by 16 genera and/or species, anaerobic – by 11 genera and/or species. Among aerobic taxa (Table), coagulase-negative Staphylococci (CoNS) were revealed in all cases. The spectrum of isolated causative pathogens was distributed along the vector Enterococcus spp. (44.3%) → Staphylococcus aureus (26.9%) → Enterobacteriaceae (13.4%). Eubacterium spp. dominated in the anaerobic microbiota cluster.
The level of EPS infestation ranged from 102 to 106 CFU/ml, Me-value ≥ 103 CFU/ml was observed in 7 (25.9%) taxa. However, for 21 taxa (77.8%), in some cases, EPS infestation ≥ 103 CFU/ml was recorded. Bacterial antibiotic susceptibility to levofloxacin ranged from 16.7 (95% CI 3.01 to 56.35) to 33.3% (95% CI 6.15 to 79.23).
Table. Identification rate and microbial load of expressed prostate secretion
|
Microorganisms |
Identification frequency, % |
Microbial load, CFU/ml |
|||
|
Median |
SD |
min |
max |
||
|
CoNS: |
100.0 |
2.00 |
0.91 |
2.00 |
4.60 |
|
S. lentus |
40.0 |
2.00 |
0.72 |
2.00 |
5.00 |
|
S. haemolyticus |
28.8 |
2.00 |
1.36 |
2.00 |
6.00 |
|
S. warneri |
26.9 |
2.00 |
0.83 |
2.00 |
5.00 |
|
S. saprophyticus |
13.5 |
2.00 |
0.49 |
2.00 |
3.00 |
|
S. epidermidis |
5.8 |
2.00 |
1.15 |
2.00 |
4.00 |
|
Enterobacteriaceae: |
13.4 |
4.00 |
1.97 |
2.00 |
6.00 |
|
E. coli |
11.5 |
3.00 |
1.97 |
2.00 |
6.00 |
|
K. oxytoca |
1.9 |
6.00 |
– |
6.00 |
6.00 |
|
Enterococci: |
44.3 |
3.00 |
1.07 |
2.00 |
4.50 |
|
E. faecalis |
30.8 |
2.00 |
1.14 |
2.00 |
5.00 |
|
E. faecium |
7.7 |
4.00 |
1.00 |
2.00 |
4.00 |
|
Enterococcus spp. |
5.8 |
2.00 |
2.31 |
2.00 |
6.00 |
|
Corynebacterium spp |
71.2 |
3.00 |
1.04 |
2.00 |
5.00 |
|
S. aureus |
26.9 |
2.00 |
0.63 |
2.00 |
4.00 |
|
Micrococcus spp. |
7.7 |
2.00 |
0.50 |
2.00 |
3.00 |
|
Streptococcus spp. |
3.8 |
3.50 |
2.12 |
2.00 |
5.00 |
|
Anaerobes: |
100.0 |
2.37 |
0.89 |
2.00 |
3.5 |
|
Eubacterium spp. |
34.6 |
4.00 |
1.31 |
2.00 |
6.00 |
|
Propionibacterium spp. |
25.0 |
2.00 |
0.97 |
2.00 |
5.00 |
|
Peptococcus spp. |
23.1 |
2.00 |
0.39 |
2.00 |
3.00 |
|
Veillonella spp. |
21.2 |
2.00 |
0.90 |
2.00 |
5.00 |
|
Peptostreptococcus spp. |
9.6 |
4.00 |
1.52 |
2.00 |
5.00 |
|
Megasphaera spp. |
7.7 |
2.00 |
0.50 |
2.00 |
3.00 |
|
Bifidobacterium spp. |
5.8 |
2.00 |
0.58 |
2.00 |
3.00 |
|
Prevotella spp. |
1.9 |
2.00 |
– |
2.00 |
2.00 |
|
Bacteroides spp. |
1.9 |
2.00 |
– |
2.00 |
2.00 |
|
Fusobacterium spp. |
1.9 |
2.00 |
– |
2.00 |
2.00 |
|
Mobiluncus spp. |
1.9 |
2.00 |
– |
2.00 |
2.00 |
|
Candida spp.: |
5.8 |
2.00 |
– |
2.00 |
2.00 |
|
C. albicans |
3.8 |
2.00 |
– |
2.00 |
2.00 |
|
C. glabrata |
1.9 |
2.00 |
– |
2.00 |
2.00 |
Note. CoNS — coagulase-negative Staphylococci; CFU/ml — colony-forming units per milliliter; SD — standard deviation
The assessment of the results of treatment with the NIH-CPSI scale revealed that after 1 month of therapy in all groups, there was a decrease in the mean sum score with statistically significant differences between groups 1, 2, and 3 (p < 0.05) (Fig. 1). After one month from the beginning of therapy, the subjective symptoms of prostatitis decreased in group 1 patients from 16.3 ± 2.5 to 8.3 ± 1.0 points (p < 0.05). Three months after the completion of therapy, this index was 6.8 ± 1.0, and six months after, it increased compared to the previous one to 7.8 ± 0.5 (p < 0.05).
In group 2, the studied index before treatment corresponded to 16.1 ± 0.7, one month after, it was 7.3 ± 0.5, three months after, it was 7.2 ± 0.8, and by six months, it decreased to 2.2 ± 0.2 points (p < 0.05). In group 3, the mean sum score before treatment was 15.8 ± 1.2, one month after, it was 6.1 ± 0.5, three months after, it was 5.6 ± 0.8, and by six months, it decreased to 2.6 ± 0.5 points (p < 0.05).
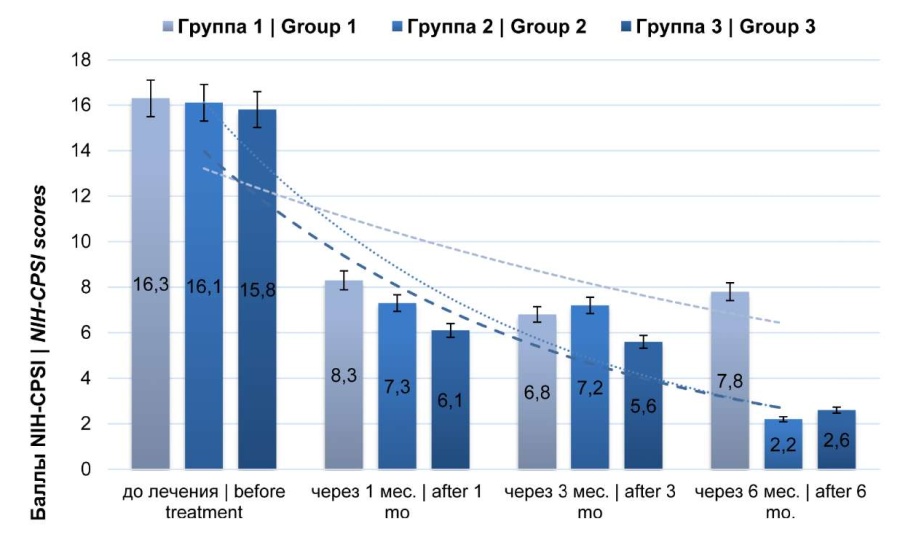
Figure 1. Dynamics of clinical manifestations (NIH-CPSI)
The IPSS symptom assessment showed the mean pre-treatment sum score was 5.3 ± 1.0 in group 1, 8.5 ± 1.0 in group 2, and 7.0 ± 1.0 in group 3. One and three months later, the index studied decreased in all groups. However, by six follow-up months, in group 1, this index tended to increase (p > 0.05) to 3.1 ± 1.0 compared to the same index after one and three months, and in groups 2 and 3, it tended to decrease to 0.9 ± 0.5 and 1.1 ± 0.5 points, respectively (p < 0.05) (Fig. 2).
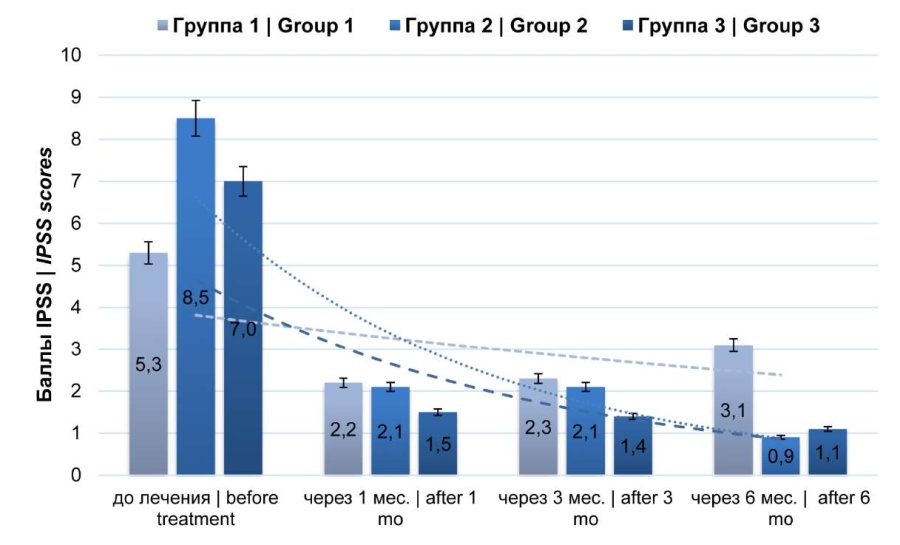
Figure 2. Dynamics of clinical manifestations (IPSS only)
The IPSS symptom assessment showed the mean pre-treatment sum score was 5.3 ± 1.0 in group 1, 8.5 ± 1.0 in group 2, and 7.0 ± 1.0 in group 3. One and three months after, the studied index decreased in all groups. However, by 6 months of observation, this index in group 1 tended to increase (p > 0.05) to 3.1 ± 1.0 compared to the same index after one and three months, and in groups 2 and 3, it tended to decrease to 0.9 ± 0.5 and 1.1 ± 0.5 points, respectively (p < 0.05) (Fig. 2).
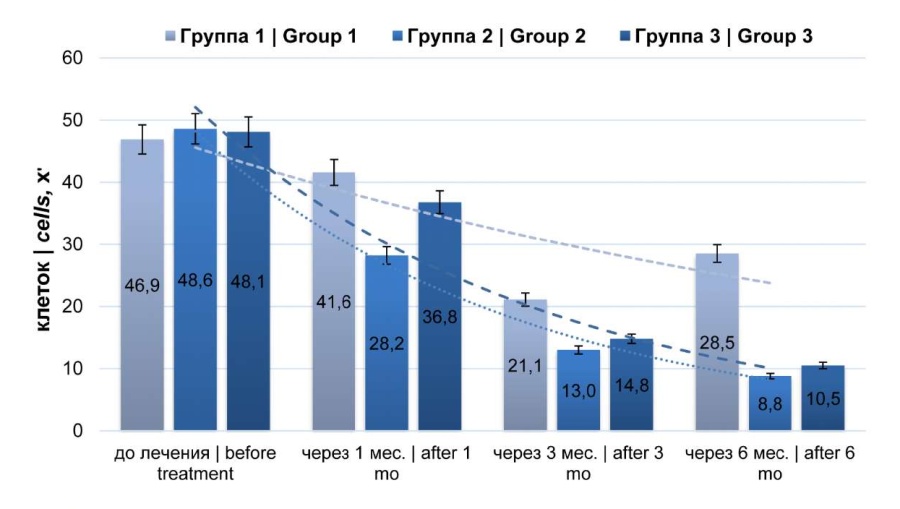
Figure 3. Dynamics of leukocyte count (microscopy of expressed prostate secretion)
Analysis of the average values of the macrophage count in patients from the studied groups revealed multidirectional trends (Fig. 4). In group 1, the studied index decreased significantly after one month (p < 0.05), remained unchanged after three months (2.2 ± 0.2/x'), and at six months, reached values almost equal to those before treatment. In contrast, in groups 2 and 3, the studied index increased (p < 0.05) one month afterward and progressively decreased three and six months afterward.
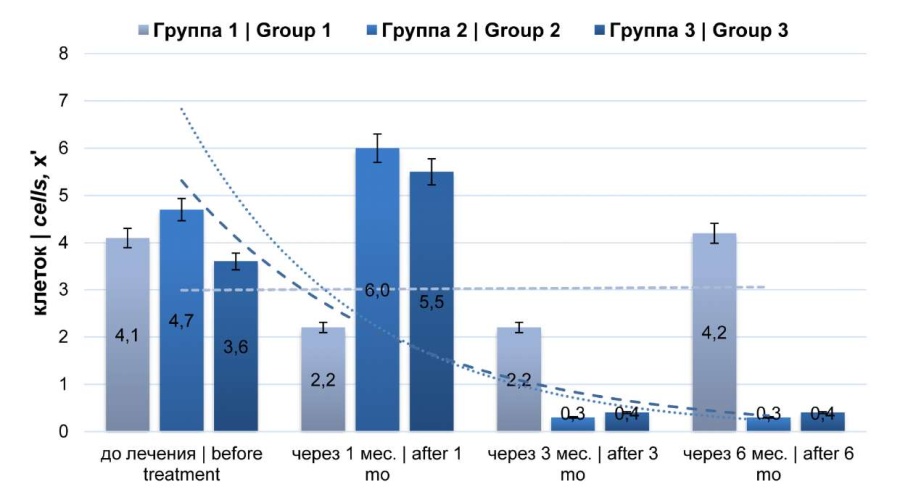
Figure 4. Dynamics of macrophages count (microscopy of expressed prostate secretion)
Uroflowmetry results showed an improvement in the maximum urinary flow rate (Q max) after 1 and 3 months of observation in all groups (Fig. 5). Six months after in group 1, this index slightly decreased (p > 0.05), in group 3, it also decreased compared to the analogous indexes after 1 and 3 months, but it was slightly higher than the initial data (p > 0.05). In group 2, after six months, the Q max values were significantly higher (p < 0.05) compared to those for the entire observation period. Despite the identified variations, in all points of observation, the Qmax values corresponded to the reference values.
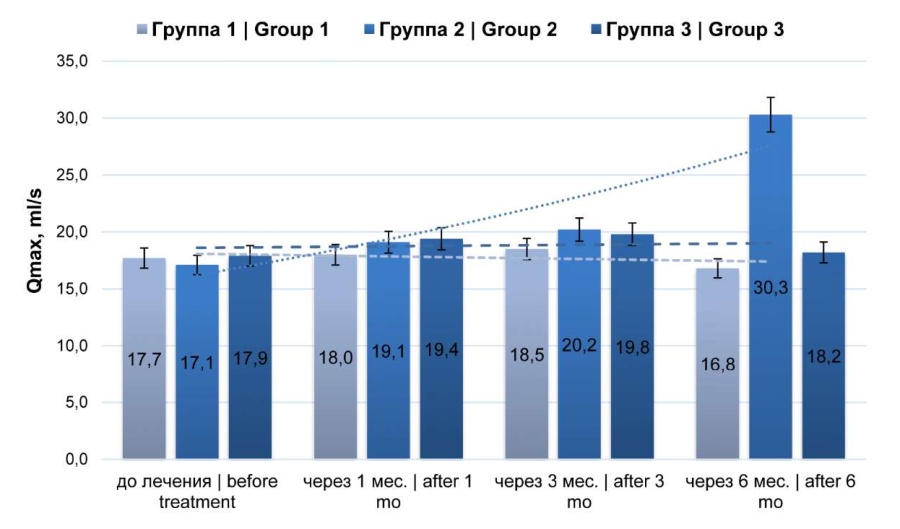
Fig. 5. Dynamics of the maximum (Qmax) urine flow rate (uroflowmetry)
According to TRUS, the mean prostate volume tended to decrease (p > 0.05) (Fig. 6).
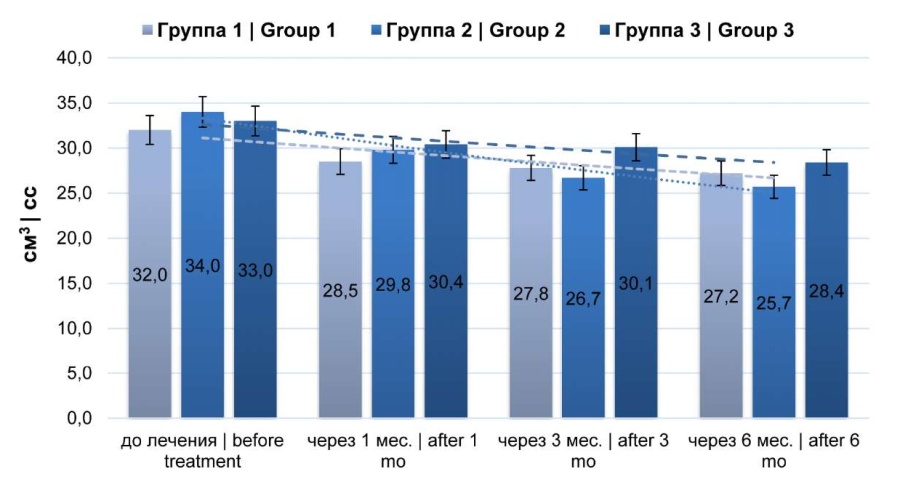
Fig. 6. Dynamics of prostate volume (transrectal prostate ultrasound)
Discussion
CRBP is a disease that occurs more frequently at a young age, and in some cases, is characterized by a recurrent course. This condition is resistant to currently accepted therapeutic options [14].
In addition, according to the available publications, the antibacterial treatment recommended by ESU and RSU as first-line therapy is not effective in all patients [15][16].
The leading causes of decreased ABT efficacy and disease recurrence are both the growth of microbial resistance to ABP and the dysfunction of immunological links of prostate defense, which are currently aggravated in patients with a history of COVID-19. It is known that one of the initial directions of treatment for COVID-19 was the use of prolonged empiric combined ABT, which caused the growth of microbial resistance. In addition, the SARS-CoV-2 suppresses the functional activity of the immunological parts of many organs and systems, which leads to the activation of persistent and opportunistic infections, including those in the prostate. These circumstances lead to the emergence of multidrug-resistant and pan-resistant superbacilli and many new variants of mixed infections, which is a real threat for this category of patients and, as a result, the search for alternative methods of CRBP treatment [17].
The efficacy of recombinant interferon α-2b combination therapy in CRBP-patients in combination with antibiotics was previously evaluated and proven. ABT of CRBP in combination with immunoactive therapy showed a clinical efficacy of up to 70.9% [9].
The present study investigated the efficacy of recombinant interferon α-2b in CRBP-patients and COVID-19 against the background of multiple antibiotic-resistant microorganisms verified in EPS. The authors showed that the treatment results of CRBP-patients with recombinant interferon α-2b were comparable with the results of treatment with antibacterial drugs. Furthermore, this study was the first to demonstrate the efficacy of recombinant interferon α-2b both in monotherapy and in combination with ABT.
The study results showed that recombinant interferon α-2b was effective, as it led to activation of the macrophages count in EPS, and after the completion of therapy, there was a registered normalization of not only the leukocytes count but also the macrophages count in EPS.
Conclusion
The inclusion of recombinant interferon α-2b with an antioxidant complex (vitamins E and C) in the standard therapy of CRBP allows for the normalization of both clinical and laboratory parameters of CRBP in most patients. The use of this drug may be one of the alternative therapy methods for patients with this pathology after COVID-19 with multidrug antibiotic resistance of microorganisms, verified in EPS.
References
1. Krupin V.N., Belova A.N., Krupin A.V. Treatment of patients with chronic bacterial prostatitis. Urology Herald. 2019;7(1):26-37. (In Russ.) https://doi.org/10.21886/2308-6424-2019-7-1-26-37
2. Breusoff А.А., Кulchavenya Е.V., Cherednichenko А.G., Stovbun S.V. What does abacterial prostatitits hide? Urology Herald. 2017;5(2):34-41. (In Russ.) https://doi.org/10.21886/2308-6424-2017-5-2-34-41
3. Zaitsev A.V., Pushkar D.Yu., Khodyreva L.A., Dudareva A.A. Bacterial prostatitis and prostatic fibrosis: modern view on the treatment and prophylaxis. Urologiia. 2016;(4):114-121. (In Russ.) EDN: XBKFCD
4. Tkachuk V.N., Tkachuk I.N., Borovec S.Ju. The results of 12-year study of the efficacy of Vitaprost in patients with chronic prostatitis. Urologicheskie vedomosti. 2016;6(4):5-9. (In Russ.) https://doi.org/10.17816/uroved645-9
5. Ibishev H.S., Chernyj A.A., Kogan M.I. Clinical features of a chronic bacterial prostatitis against deficiency of testosterone. Urology Herald. 2013;(1):39-45. (In Russ.) https://doi.org/10.21886/2308-6424-2013-0-1-39-45
6. Demidko Yu.L., Gazimiev M.A., Baiduvaliev A.M., Lachinov E.L., Saenko V.S. The use of vitaprost preparations in the treatment of patients with prostate diseases. Urologiia. 2014;(1):62-67. (In Russ.) EDN: RYHWDB
7. Naboka Y.L., Kogan M.I., Gudima I.A., Chernitskaya M.L., Ibishev Kh.S., Khasigov A.V., Mitusova E.V. The role of non-clostridial anaerobes in the development of infectious and inflammatory diseases of the urinary and reproductive systems. Urologiia. 2013;(6):118-121. (In Russ.) EDN: RTOFXL
8. Kogan M.I., Naboka Yu.L., Ibishev Kh.S., Gudima I.A. The non-sterility of the urine of a healthy person is a new paradigm in medicine. Urologiia. 2014;(5):48-52. (In Russ.) EDN: TFDOIP
9. Ibishev Kh.S., Krainy P.A., Mantsov A.A. Efficacy of recombinant interferon alpha-2b in the treatment of chronic recurrent bacterial prostatitis. Urologiia. 2020;(4):21-26. (In Russ.) https://doi.org/10.18565/urology.2020.4.21-26
10. Kuzmenko A.V., Kuzmenko V.V., Gyaurgiev T.A. Post-covid complications in urology and their prevention. Urologiia. 2020;(3):154-159. (In Russ.) https://doi.org/10.18565/urology.2022.3.154-159
11. Kotov S.V., Pulbere S. A., Alesina N.V., Boyarkin V.S., Guspanov R.I., Belomytsev S.V., Kotova D.P. The problem of antibiotic resistance of microorganisms in patients with urinary tract infections. Urologiia. 2021;(1):5-12. (In Russ.) https://doi.org/10.18565/urology.2021.1.5-12
12. Shangichev A.V., Naboka Yu.L., Ibishev Kh.S., Kogan M.I. The microbical spectrum and antibiotic sensitivity of microorganisms of prostate secretion in chronic bacterial prostatitis. Kuban scientific medical bulletin. 2010;(3-4):207-211. (In Russ.) EDN: MUMCOJ
13. Kogan M.I., Naboka Yu.L., Ismailov R.S. Prostate secretion microbiota: a comparative analysis of category II and IIIA chronic prostatitis. Urology. 2020;(2):16-22. (In Russ.) https://doi.org/10.18565/urology.2020.2.16-22
14. Kulchavenya E.V., Brizhatyuk E.V., Holtobin D.P., Cherednichenko A.G. A modern approach to the diagnosis of chronic prostatitis. Urologiia. 2021;(2):32-39. (In Russ.) https://doi.org/10.18565/urology.2021.2.32-39
15. Krainii P.A., Ibishev K.S. Evaluation by electron microscopy of immunological disorders in the secretion of the prostate gland of patients with chronic recurrent bacterial prostatitis. Urologiia. 2021;(4):68-72. (In Russ.) https://doi.org/10.18565/urology.2021.4.68-72
16. Naboka Yu.L., Kogan M.I., Chernickaia M.l., Gudima I.A., Ibishev Kh.S., Ferzauli Kh.A. Microbial spectrum of prostate secretion and persistence factors of bacteria found in chronic bacterial prostatitis. Biulliuten’ Orenburgskogo nauchnogo centra UrO RAN. 2012;(3):11. (In Russ.) EDN: REYPGN
17. Ibishev Kh.S., Mamedov E.A., Gusova Z.R., Palenyy A.I., Prokop Y.O. Serum testosterone and testicular hemodynamics before and after infection with SARS-CoV-2 (pilot study). Urologiia. 2021;(5):5-9. (In Russ.) https://doi.org/10.18565/urology.2021.5.5-9
About the Authors
Kh. S. IbishevRussian Federation
Khalid S. Ibishev — M.D., Dr.Sc.(Med), Assoc.Prof. (Docent), Prof., Dept. of Urology and Human Reproductive Health (with the Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
Yu. L. Naboka
Russian Federation
Yulia L. Naboka — M.D., Dr.Sc.(Med), Full Prof., Head, Dept. of Microbiology and Virology No.1, Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
P. A. Krainiy
Russian Federation
Pavel A. Krainiy — M.D., Cand.Sc.(Med), Urologist, Medical Center “Professional”, Ltd.
47/4 26th line St., Rostov-on-Don, 344037
Competing Interests:
The authors declare no conflict of interests
I. A. Gudima
Russian Federation
Irina A. Gudima — M.D., Dr.Sc.(Med), Assoc.Prof. (Docent), Prof., Dept. of Microbiology and Virology No.1, Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
A. D. Plyakin
Russian Federation
Alexandr D. Plyakin — M.D., Urologist, Urology Division, Lenin Shakhty City Emergency Hospital, Shakhty, Russian Federation.
153 Shevchenko St., Rostov Region, Shakhty, 346500
Competing Interests:
The authors declare no conflict of interests
J. O. Prokop
Russian Federation
Jan O. Prokop — M.D, Urologist, Postgrad. student, Dept. of Urology and Human Reproductive Health (with the Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
A. V. Ilyash
Russian Federation
Anna V. Ilyash — M.D., Cand.Sc.(Med), Assist.Prof., Dept. of Urology and Human Reproductive Health (with the Pediatric Urology and Andrology Course), Rostov State Medical University.
29 Nakhichevanskiy Ln., Rostov-on-Don, 344022
Competing Interests:
The authors declare no conflict of interests
Review
For citations:
Ibishev Kh.S., Naboka Yu.L., Krainiy P.A., Gudima I.A., Plyakin A.D., Prokop J.O., Ilyash A.V. Treatment of post-COVID-19 patients with chronic recurrent prostatitis: efficacy of recombinant interferon α-2b medications. Urology Herald. 2022;10(4):32-42. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-32-42













































