Scroll to:
Opportunities for early detection of prostate cancer in young and middle-aged men
https://doi.org/10.21886/2308-6424-2022-10-1-110-120
Abstract
Prostate cancer (PCa) is a public health problem worldwide. Among all malignant tumors, PCa ranks second in prevalence (105 out of 185 countries) and fifth in cause of death in men in 46 countries. In some cases, this pathology is verified in men under the age of 50, including the advanced stage of the metastatic process. The review article provides information on the epidemiology and prevalence of PCa in young men obtained from the PubMed, CrossRef, and Scopus databases. The data on the probable causal relationship of a number of factors potentially affecting the development of prostate carcinoma are presented. Little-known and new molecular genetic changes are described, including those associated with prostate-specific antigen (PSA), with a proven role in the development of this disease, the use of which will make it possible to predict PCa development in the early stages in a timely manner. It has been determined that the common methods for diagnosing carcinoma in the population, assessing the level of serum PSA, are not always accurate and that the algorithm for their use has not been finally formed. The study of risk factors for the development of PCa in young patients will make it possible to formulate a new diagnostic approach based on considering personal molecular genetic information. The development of this direction is relevant and has an important social and economic importance, considering the study of the contingent of the able-bodied population.
For citations:
Startsev V.Yu., Shpot E.V., Karaev D.K., Krivonosov D.I. Opportunities for early detection of prostate cancer in young and middle-aged men. Urology Herald. 2022;10(1):110-120. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-110-120
Introduction
Prostate cancer (PCa) occupies one of the leading positions out of cancer diseases and causes of cancer death among men worldwide (6.6% of the total number of deaths) [1]. The number of cases of PCa diagnosed among all other types of cancer is in the leading position in 105 countries of the world. On average, annually, 1,600,000 new cases of PCa and 366,000 lethal cases caused by this disease are registered worldwide [2]. In countries with high income, the highest rates of malignant neoplasm are registered [3]. The rate of PCa lethality in some high-income regions decreases, while in countries with low and middle income, it tends to increase. The prevalence of PCa increases significantly in men who move from countries with a low risk of developing this pathology to countries with high risk [3].
Strategy of searching for publications
The authors studied available publications on the epidemiology and prevalence of PCa in young and middle-aged men obtained from the Pubmed, CrossRef, and Scopus databases and dated 1997 – 2021. Publications were searched by keywords.
Prostate cancer prevalence in age groups
In Russian men, PCa is often diagnosed after 60 years [4]. Recent observations show that PCa cases are not accidentally detected among adolescents and people younger than 50 years. The prevalence of PCa increases in patients aged 15 – 40 years and has stable growth rates, on average, of 2% from 1990 (p < 0.01) [5]. In this age group, Bleyer et al. [5] (2020) diagnosed a metastatic form of PCa six times more frequently in older men, and 5-year overall survival in PCa patients aged 40 to 80 years was 95 – 100%; 20 to 29 years — 50%; 25 to 34 years — 80% [5].
In the USA, in 2000–2015, there were cases of PCa in adolescents aged 13 and 16 years, and an increase in the prevalence of PCa in men older than 30 years (Fig. 1A, 1B) [5]. In half of patients younger than 25 years old with diagnosed prostate carcinoma, an early stage of PCa was detected, in 9% of patients younger than 40 years old, remote metastases were revealed (in comparison with 4% of older patients aged 40 – 80 years old). The share of patients with the initial stage of PCa was proportional to age of patients: >50% from 15 to 24 years old, 10% from 25 to 29 years old, 6% from 35 to 39 years old, and 2% from 40 to 80 years old (Fig. 1C).
Fig. 1 contains the data of The Surveillance, Epidemiology, and End Results Program on the incidence rate of prostate carcinoma in 17 USA Regions in 2000 – 2015. The age range of adolescents and young adults (AYA) is highlighted in yellow (1A) among men aged 10 to 84 years old; (1B) among men aged 10 to 39 years old; (1C) depending on the degree of the disease when diagnosed in AYA and senior men.
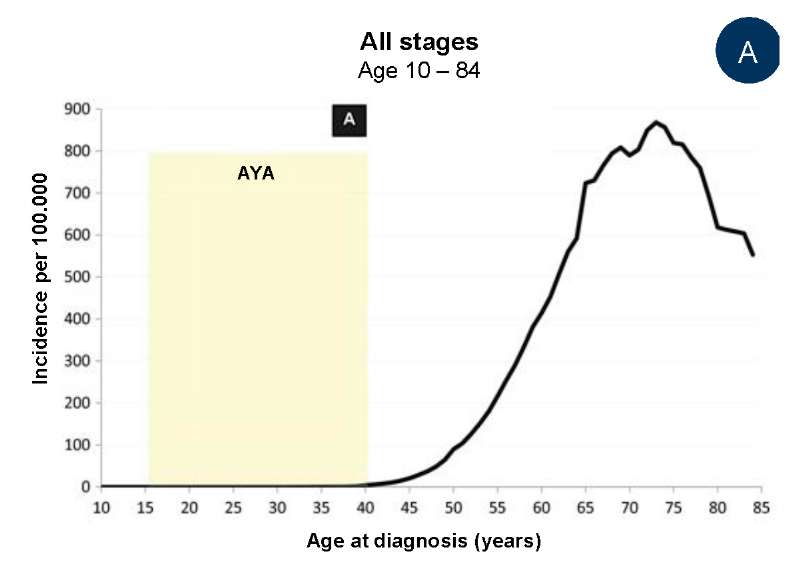
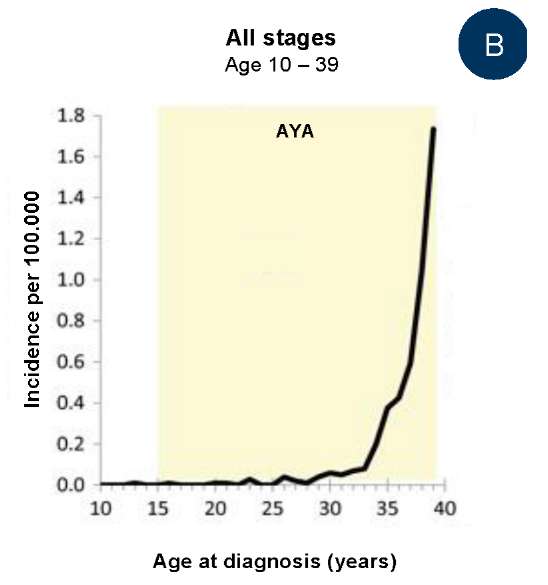
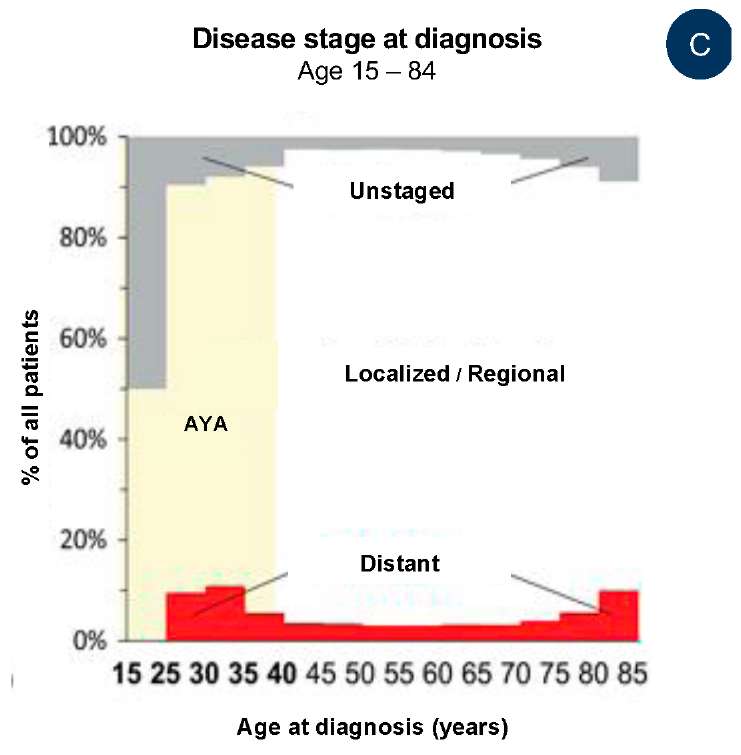
Fig. 1. The frequency of prostate cancer detection:
A — among men aged 10 to 84 years (the age range of adolescents
and young people (AYA) is highlighted in yellow);
B — among men aged 10 to 39 years;
C — considering the grade in various age groups at the time of diagnosis [5]
The results of numerous epidemiologic studies showed that the lowest level of PCa prevalence was observed in Central and Eastern Europe and Asia. The lethality rate of this disease decreased in Japanese and Israel populations in 2007–2016. Simultaneously, it increased in the population of Thailand, Kyrgyzstan, and Uzbekistan [6]. According to the International Agency for Research on Cancer, the prevalence of PCa in men aged 45 – 54 years did not tend to decrease in 43 populations on five continents within a 24-year observation [7].
Thus, the rate of PCa diagnostics in men < 50 years old continues to increase, but the causes of this phenomenon are still unclear. Researchers report an increase in the actual risk of pathological development. An increase in the rate of screening studies on PCa could lead to some increase in the prevalence of the disease. Men < 50 years of age are not a target group for PCa testing, which leads to a decrease in PCa detection in young men. However, the screening of the general population is unlikely to solve this issue either. It is necessary to look for alternative diagnostic methods with special criteria for choosing patients to avoid hyperdiagnostics of low aggressive forms of tumor and increase in economic burden.
The clinical peculiarities of prostate cancer depend on the age of the patients.
Compared with the situation in patients of traditionally senior age (> 60 years old), most prostate tumors in young men are detected in localized stages, with a low risk of progression and relatively low secondary lesion of the regional lymph nodes [8].
According to the results of another study, during a 17-year observation (on average, 79.6 months; from 4.5 to 98.2 months), PCa was diagnosed in 4,716 patients (including 29 patients < 50 years old) [9]. In 26 (89.7%) men, adenocarcinoma was histologically verified, which was 0.55% of all detected carcinomas. In three patients from this group, embryonal rhabdomyosarcoma and stromal sarcoma were diagnosed. The mean age of the patients in the main PCa group diagnosed primarily was 46.8 ± 2.8 years (39 – 50 years). During the examination, transrectal ultrasound showed that the mean prostate volume in the patients was 29.33 ± 10.0 ml. In nine patients, digital rectal examination revealed suspicious areas in the prostate. In other patients, pathologically dense areas were not detected. In 7 (29%) patients, the most frequent symptoms were low urinary tract symptoms; in 3 (13%) patients — pain in bones; and in 3 (13%) patients — hematuria (Table). In 6 (23%) patients from the study group, a family medical history of these diseases was revealed.
Table. Symptoms and signs of prostate cancer development in men of different age categories [9]
|
Categories of patients |
Age of patients with PCa, years |
p |
|
|
younger 50 years (n = 26) |
over 50 years (n = 108) |
||
|
Age, years |
47.0 ± 2.7 |
75.13 ± 8.1 |
|
|
Digital rectal examination |
0.191 |
||
|
positive |
8 (47) |
43 (62) |
|
|
negative |
9 (53) |
26 (38) |
|
|
Prostate volume (TRUS), ml |
29.33 ± 10.0 |
37.10 ± 17.9 |
0.122 |
|
PSA, ng/ml |
0.847 |
||
|
< 4, n (%) |
0 (0) |
5 (6) |
|
|
4 – 10, n (%) |
7 (32) |
25 (32) |
|
|
10 – 20, n (%) |
5 (23) |
18 (23) |
|
|
> 20, n (%) |
10 (45) |
30 (39) |
|
|
Initial indicators |
0.011 |
||
|
LUTS |
7 (29) |
50 (63) |
|
|
randomly installed |
9 (38) |
10 (13) |
|
|
bone pain |
3 (13) |
3 (4) |
|
|
hematuria |
3 (13) |
5 (6) |
|
|
dysuria |
2 (7) |
3 (4) |
|
|
acute urinary retention |
0 (0) |
5 (6) |
|
|
others |
0 (0) |
3 (4) |
|
|
Stage: |
0.652 |
||
|
I, n (%) |
0 (0) |
5 (6) |
|
|
II, n (%) |
14 (56) |
38 (48) |
|
|
III, n (%) |
4 (16) |
13 (17) |
|
|
IV, n (%) |
7 (28) |
23 (29) |
|
|
Risk stratification a |
0.678 |
||
|
low |
2 (12) |
17 (22) |
|
|
average |
7 (44) |
29 (37) |
|
|
high |
7 (44) |
33 (42) |
|
|
Treatment b |
0.001 |
||
|
surgical |
13 (59) |
14 (18) |
|
|
non-surgical |
9 (41) |
62 (82) |
|
Notes:
- PCa — prostate cancer; TRUS — transrectal ultrasound;
PSA — prostate-specific antigen; LUTS — lower urinary tract symptoms - Group a: according to the D'Amico risk classification, for localized / locally advanced disease;
disseminated tumors are excluded.
Group b: includes hormonal deprivation, radiation therapy, watchful waiting
Gupta et al. (2017) showed that PCa with an early onset (in men < 55 years old) was considered a clinical disease that is different from a disease diagnosed in senior men [10].
Some large population studies demonstrated a low survival in patients < 50 years old with advanced PCa or unidentified tumor stage compared to older patients. The level of prostate-specific antigen (PSA) in these patients was lower due to low-grade prostate adenocarcinoma [11]. It was concluded that in patients with low-grade carcinoma, the level of PSA was not correlated with the stage of the tumor. Patients 35 – 44 years old with a high degree of malignancy (8 – 10 Gleason score) had a higher risk of lethality compared to patients 65 – 74 years old. The genetic component was also closely associated with the early onset of PCa.
Factors that can affect the development of prostate cancer
An important factor in the early onset of PCa is a burdened family medical history with a dominant gene inherited through the parental lineage.
Currently, new variants of this disease are revealed that are genome-associated with PSA [13]. In a two-stage study by Schaid et al. (2021), the first stage revealed potential risk alleles in men with PCa with burdened medical history (491 cases of PCa + 429 control cases of prostate hyperplasia, BPH). A multifactorial analysis was based on a total Gleason score, tumor size, metastases, tumor stage, PSA level at the time of diagnosis verification, systemic recurrence, and time to death from primary PCa diagnosis. In the second stage, an individual model was used to detect some genes. The role of the activation of the genes associated with PCa was revealed. Some of them were known (ATM, BRCA2, HOXB13, FAM111A, EMSY, HNF1B, KLK3, MSMB, PCAT1, PRSS3, and TERT) and some of them were new ones (PABPC1, QK1, FAM114A1, MUC6, MYCBP2, RAPGEF4, RNASEH2B, ULK4, XPO7, and THAP3) [13].
Another study showed that long ncRNA played an important role in PCa development (AC245100.4 lncRNA) [14]. The results of the study showed that the expression of RNA was significantly elevated in tissues and PCa cell lines. Excessive expression of AC245100.4 significantly contributes to the proliferation and migration of atypical prostatic cells, while the inhibition of this RNA leads to suppression of proliferation and migration of cells, which was proved with a double analysis of oxidative enzyme luciferase and the results of RNA immunoprecipitation analysis.
The authors of the study [15] showed a significant role for genes that encode enzymes for estrogen metabolism as PCa progresses (CYP17, CYP19, COMT, CYP1B1, and UGT1A1) [15]. The study included a population of patients of African origin (Guadeloupe: PCa, n= 498; BPH, n = 565 (control group), and Democratic Republic of Congo: PCa, n = 162; BPH, n = 144) with a high risk of PCa. This allowed the researchers to identify an association between the genes that encode androgen and estrogen metabolism and PCa carcinogenesis.
An international consortium of 62 medical centers from 20 countries performed a targeted screening for PCa in men with BRCA1/2 mutations [16]. Examination of 2,481 men (791 – BRCA1 carriers, 531 – BRCA1-control; 731 – BRCA2 carriers, 428 – BRCA2-control) showed that the level of PSA was >3.0 ng/ml in 199 (8%) men. After 162 prostate biopsies, 59 cases of PCa were diagnosed (18 carriers + 10 control BRCA1; 24 carriers + 7 control BRCA2), and 66% of tumors were identified as medium and high risk of cancer progression. The positive predictive value (PPV) for biopsy with the PSA 3.0 ng/ml threshold in patients with BRCA2 was 48%, that is, two times higher than the PPV observed earlier. In BRCA2 carriers, significant differences in the identification of PCa of the medium and high risk of development were revealed.
Recent results showed that a combination of inherited PCa factors and the environmental quality index intensified the effect of each risk criterion on tumor development [17]. Exogenous factors of environmental quality index can interfere and/or change such biological processes as excretion and hormonal function, inflammation, DNA damage, and suppression / hyperexpression of genes. The analysis showed an association between environmental factors and PCa development rates (CI 34.84 – 53.54) and associated pharmacotherapy, metabolic syndrome, and inflammatory diseases of the urogenital organs.
A significant role in early PCa diagnosis is played by the peculiarities of nutrition associated with an increased content of inflammatory factors in foods (IL-6, C-peptide) and a hyperinsulinemic diet, which is associated with an increased level of glycated hemoglobin (HbA1C). Hyperinsulinemia and inflammation are two associated biological pathways that connect the diet and PCa development. A 28-year observation among 41,209 men who worked in medical institutions revealed 5,929 cases of PCa, of which 667 had a fatal outcome [18]. For each standard deviation of diet with hyperinsulinemia, the risk of PCa progression was higher by 7% (HR: 1.07; 95% CI: 1.01 – 1.15), and the risk of lethal outcome increased by 9% (HR: 1.09; 95% CI: 1.00 – 1.18). A diet with an increased content of inflammatory elements was associated with a lower risk of developing advanced PCa in a model adjusted for age. However, no significant data were obtained on these associations in patients with corrected nutrition and PCa development in the general population studied.
There are data that show a clinically significant association between the risk of the development of prostate carcinoma and the way of life of a patient at any age and his nutrition [19]. It is suggested that green tea catechins, tomato lycopene, and other products play a certain role in the reactions of the oncogenic pathway to oxidative stress. Omega-3 fatty acids, products with high saturated acid content, ellagitannin in pomegranate extract, products containing isoflavones, genistein, and diazene, vitamins and mineral supplements, selenium, etc. affect the development of PCa to a certain extent.
An association was found between the level of PSA in the groups of young fertile men and infertile men. The study included 956 (90%) infertile and 102 (10%) fertile men [20]. In each patient, the researchers analyzed the levels of PSA, sex hormones, and qualitative parameters of the sperm. Patients with infertility had higher levels of PSA, and probably, a higher risk of PCa development. The level of PSA > 1 ng/ml was observed in 32% of infertile men and 20% of fertile men. In 176 men < 40 years old (27%), the PSA level of PSA was > 1 ng/ml; out of these patients, most men were infertile (28% infertile vs 17% fertile > p = 0.03).
A total of 1,782 men were tested for the total level of PSA (t/PSA) with a further calculation of the ratio of free PSA (f/PSA) to total PSA (f/tPSA) [21]. A 30-year observation showed that the risk of death from PCa was significantly elevated in men with higher initial PSA, the risk coefficient was 1.04 (95% CI – 1.03–1.09). The authors concluded on the feasibility of reducing the rate of PSA screening or its cancellation in men aged 55 – 70 years, if the level of PSA is < 2.0 ng/ml and f/t PSA 0.25.
British researchers [22] studied the results of a 5.6-year observation of 4,575 PCa cases and established an association between race and the development of oncological processes in prostatic cells. The odds ratio of belonging to African race to European was – ¼ 2.61, 95% CI ¼ 2.10 – 3.24 and Asian to European – 0.62 (0.73 – 0.89).
Thus, it is not reasonable to take into account the only factor predisposing to PCa. An algorithm of early diagnosis for PCa is necessary that considers the multifactorial nature of the pathological development adjusted for age, environmental factors, eating habits, comorbid pathology, burdened family medical history, anthropometry, race, and individual morphogenetic profile. Currently, there is no state-scale screening for PCa for young men.
Conclusion
Diagnostics and treatment of young men (< 50 years old) with PCa have medical social and economic significance. A complex of new molecular, genetic, and histological methods of study is established for early PCa diagnosis. However, these methods did not become widespread in routine clinical practice. Primarily, it is associated with a lack of sampling among young men and a high cost of proposed genetic studies, leading to late tumor diagnosis in men of a high-risk group and untimely treatment.
It is necessary to develop standardized examination algorithms based on the results of molecular genetic studies in combination with individual anthropometric, genetic, ecologic, race, diet, and somatic factors. Consideration of these factors will provide timely identification of the aggressive form of prostate carcinoma in young and medium-age men. This will allow for the preservation of the employability of the male population of reproductive age and manage the medical costs of the expected treatment.
References
1. Sadeghi-Gandomani HR, Yousefi MS, Rahimi S, Yousefi SM, Karimi-Rozveh A, Hosseini S, Mahabadi AA, Abarqui HF, Borujeni NN, Salehiniya H. The incidence, risk factors, and knowledge about the prostate cancer through worldwide and Iran. WCRJ. 2017;4(4):e972. DOI: 10.32113/wcrj_201712_972.
2. Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17-18):1105-1140. DOI: 10.1101/gad.315739.118.
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27. DOI: 10.1158/1055-9965.EPI-15-0578.
4. Kaprin A.D., Starinsky V.V., Shakhzadova A.O. The state of oncological care to the population of Russia in 2019. Moscow: P.A. Herzen Institute of Oncology - branch of the FSBI "NMIC of Radiology" Ministry of Health of Russia; 2020.
5. Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer. 2019;126(1):46-57. DOI: 10.1002/cncr.32498.
6. Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, Zheng Y, Ye D. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol. 2021;18(5):282-301. DOI: 10.1038/s41585-021-00442-8.
7. Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB, Devesa SS. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388-400. DOI: 10.1002/ijc.29894.
8. Macneil J, Maclean F, Delprado W. Pathological Characteristics of Prostate Cancer Occurring in Younger Men: A Retrospective Study of Prostatectomy Patients. Urology. 2019;134:163-167. DOI: 10.1016/j.urology.2019.08.048.
9. Huang TH, Kuo JY, Huang YH, Chung HJ, Huang WJ, Wu HH, Chang YH, Lin AT, Chen KK. Prostate cancer in young adults-Seventeen-year clinical experience of a single center. J Chin Med Assoc. 2017;80(1):39-43. DOI: 10.1016/j.jcma.2016.10.004.
10. Gupta S, Gupta A, Saini AK, Majumder K, Sinha K, Chahal A. Prostate Cancer: How Young is too Young? Curr Urol. 2017;9(4):212-215. DOI: 10.1159/000447143.
11. McGuire BB, Helfand BT, Loeb S, Hu Q, O'Brien D, Cooper P, Yang X, Catalona WJ. Outcomes in patients with Gleason score 8-10 prostate cancer: relation to preoperative PSA level. BJU Int. 2012;109(12):1764-9. DOI: 10.1111/j.1464-410X.2011.10628.x
12. Sartor O. Why is prostate cancer incidence rising in young men? Cancer. 2019;126(1):17-18. DOI: 10.1002/cncr.32497.
13. Schaid DJ, McDonnell SK, FitzGerald LM, DeRycke L, Fogarty Z, Giles GG, MacInnis RJ, Southey MC, Nguyen-Dumont T, Cancel-Tassin G, Cussenot O, Whittemore AS, Sieh W, Ioannidis NM, Hsieh CL, Stanford JL, Schleutker J, Cropp CD, Carpten J, Hoegel J, Eeles R, Kote-Jarai Z, Ackerman MJ, Klein CJ, Mandal D, Cooney KA, Bailey-Wilson JE, Helfand B, Catalona WJ, Wiklund F, Riska S, Bahetti S, Larson MC, Cannon Albright L, Teerlink C, Xu J, Isaacs W, Ostrander EA, Thibodeau SN. Two-stage Study of Familial Prostate Cancer by Whole-exome Sequencing and Custom Capture Identifies 10 Novel Genes Associated with the Risk of Prostate Cancer. Eur Urol. 2021;79(3):353-361. DOI: 10.1016/j.eururo.2020.07.038.
14. Xie H, Zhao J, Wan J, Zhao J, Wang Q, Yang X, Yang W, Lin P, Yu X. Long non‑coding RNA AC245100.4 promotes prostate cancer tumorigenesis via the microRNA‑145‑5p/RBBP5 axis. Oncol Rep. 2021;45(2):619-629. DOI: 10.3892/or.2020.7894.
15. Brureau L, Moningo D, Emeville E, Ferdinand S, Punga A, Lufuma S, Blanchet P, Romana M, Multigner L. Polymorphisms of Estrogen Metabolism-Related Genes and Prostate Cancer Risk in Two Populations of African Ancestry. PLoS One. 2016;11(4):e0153609. DOI: 10.1371/journal.pone.0153609.
16. Bancroft EK, Page EC, Castro E, Lilja H, Vickers A, Sjoberg D, Assel M, Foster CS, Mitchell G, Drew K, Mæhle L, Axcrona K, Evans DG, Bulman B, Eccles D, McBride D, van Asperen C, Vasen H, Kiemeney LA, Ringelberg J, Cybulski C, Wokolorczyk D, Selkirk C, Hulick PJ, Bojesen A, Skytte AB, Lam J, Taylor L, Oldenburg R, Cremers R, Verhaegh G, van Zelst-Stams WA, Oosterwijk JC, Blanco I, Salinas M, Cook J, Rosario DJ, Buys S, Conner T, Ausems MG, Ong KR, Hoffman J, Domchek S, Powers J, Teixeira MR, Maia S, Foulkes WD, Taherian N, Ruijs M, Helderman-van den Enden AT, Izatt L, Davidson R, Adank MA, Walker L, Schmutzler R, Tucker K, Kirk J, Hodgson S, Harris M, Douglas F, Lindeman GJ, Zgajnar J, Tischkowitz M, Clowes VE, Susman R, Ramón y Cajal T, Patcher N, Gadea N, Spigelman A, van Os T, Liljegren A, Side L, Brewer C, Brady AF, Donaldson A, Stefansdottir V, Friedman E, Chen-Shtoyerman R, Amor DJ, Copakova L, Barwell J, Giri VN, Murthy V, Nicolai N, Teo SH, Greenhalgh L, Strom S, Henderson A, McGrath J, Gallagher D, Aaronson N, Ardern-Jones A, Bangma C, Dearnaley D, Costello P, Eyfjord J, Rothwell J, Falconer A, Gronberg H, Hamdy FC, Johannsson O, Khoo V, Kote-Jarai Z, Lubinski J, Axcrona U, Melia J, McKinley J, Mitra AV, Moynihan C, Rennert G, Suri M, Wilson P, Killick E; IMPACT Collaborators, Moss S, Eeles RA. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-99. DOI: 10.1016/j.eururo.2014.01.003.
17. Jagai JS, Messer LC, Rappazzo KM, Gray CL, Grabich SC, Lobdell DT. County-level cumulative environmental quality associated with cancer incidence. Cancer. 2017;123(15):2901-2908. DOI: 10.1002/cncr.30709. Erratum in: Cancer. 2019;125(10):1756. PMID: 28480506; PMCID: PMC6121813.
18. Fu BC, Tabung FK, Pernar CH, Wang W, Gonzalez-Feliciano AG, Chowdhury-Paulino IM, Clinton SK, Folefac E, Song M, Kibel AS, Giovannucci EL, Mucci LA. Insulinemic and Inflammatory Dietary Patterns and Risk of Prostate Cancer. Eur Urol. 2021;79(3):405-412. DOI: 10.1016/j.eururo.2020.12.030.
19. Zuniga KB, Chan JM, Ryan CJ, Kenfield SA. Diet and lifestyle considerations for patients with prostate cancer. Urol Oncol. 2020;38(3):105-117. DOI: 10.1016/j.urolonc.2019.06.018.
20. Boeri L, Capogrosso P, Cazzaniga W, Ventimiglia E, Pozzi E, Belladelli F, Schifano N, Candela L, Alfano M, Pederzoli F, Abbate C, Montanari E, Valsecchi L, Papaleo E, Viganò P, Rovere-Querini P, Montorsi F, Salonia A. Infertile Men Have Higher Prostate-specific Antigen Values than Fertile Individuals of Comparable Age. Eur Urol. 2021;79(2):234-240. DOI: 10.1016/j.eururo.2020.08.001.
21. Lundgren PO, Kjellman A, Norming U, Gustafsson O. Association between one-time prostate-specific antigen (PSA) test with free/total PSA ratio and prostate cancer mortality: A 30-year prospective cohort study. BJU Int. 2021;128(4):490-496. DOI: 10.1111/bju.15417.
22. Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, Travis RC. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117(10):1562-1571. DOI: 10.1038/bjc.2017.312.
About the Authors
V. Yu. StartsevRussian Federation
Vladimir Yu. Startsev — M.D., Dr.Sc.(Med), Full Prof.; Prof., Dept. of Oncology, Pediatric Oncology and Radiation Therapy; Head, Dept. of Urology
St. Petersburg
E. V. Shpot
Russian Federation
Evgeniy V. Shpot — M.D., Dr.Sc.(Med), Full Prof.; Prof., Institute of Urology and Reproductive Health; Head, Urology Division
Moscow
D. K. Karaev
Russian Federation
Jahandar K. Karaev — M.D.; Urologist, Urology Division, University Clinical Hospital No. 2
Moscow
D. I. Krivonosov
Russian Federation
Dmitry I. Krivonosov — M.D.; Urologist
St. Petersburg
Review
For citations:
Startsev V.Yu., Shpot E.V., Karaev D.K., Krivonosov D.I. Opportunities for early detection of prostate cancer in young and middle-aged men. Urology Herald. 2022;10(1):110-120. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-110-120













































