Scroll to:
A new approach to use of oral mucosa in reconstructive urethral surgery: micrografts
https://doi.org/10.21886/2308-6424-2022-10-1-70-83
Abstract
Introduction. Treatment of patients with complex urethral strictures is an actual problem of reconstructive urology. After multi-staged urethral surgery with multiple revisions the new reconstruction is limited by paucity of plastic material (for grafts and flaps). In-thing, new materials for urethral reconstruction (various auto, allo and xenografts) are still being developed in reconstructive urethral surgery.
Purpose of the study. To study the possibility of using oral mucosa micrografts to form the urethral plate in the multi-stage surgery for patients with extended urethral strictures.
Materials and methods. In the experimental study, male Wistar rats (22 individuals) weighing 300 – 400 g underwent a full-thickness skin wound after intramuscular sedation. We used the wound chamber (12 mm diameter) to exclude the wound contraction. The oral mucosa graft was harvested (6 mm in diameter). After pre-fabrication, the graft was minced to fragments < 1 mm2. Micrografts with fibrin-thrombin glue were applied to the wound. By day 45, the epithelial plate was excised for histological examination. In the clinical study, 4 patients with recurrent penile urethral strictures were treated with staged urethroplasty with urethral plate formation using oral mucosa micrografts. The average length of the stricture was 7.5 ± 1.2 cm (with extremely narrow and obliteration sites). The urethral plate was formed as the first stage. The preparation of the graft bed and oral mucosa grafts harvesting was carried out according to the standard procedure. Micrografts preparation and implantation was carried out as in experimental part of this study. After 6 months, neourethra tubularization was performed. The patients were evaluated every 3 months after the final stage of urethroplasty (uroflowmetry, ultrasound, X-ray, PROM-USS). The median follow-up was 9 months (3 – 18 months).
Results. On day 15, in the experimental study, in 16 of 22 (72.7%) rats, the wound chambers had focal growth of the oral mucosa epithelium. On day 45, the wounds healed completely healed with oral mucosa. The final area of the plate was 78 ± 12 mm2. In the clinical study, 6 months after the first stage, all patients (n = 4) had a urethral plate covered with an oral mucosa epithelium without scar formation and sufficient for neurethra tubularization. All patients underwent urethral tubularization. After catheter removal, all men urinated. After 9 months (median follow-up, n = 3), the Qmax was 22.7 ± 4.2 ml/s, the post-void residual urine was 34.8 ± 2.2 ml, the total PROM-USS score was 7.4 ± 1.2, urethral lumen is preserved. All patients showed high satisfaction with the treatment.
Conclusion. The oral mucosa micrografts showed good take in heterotopic transplantation (72.7%) with the formation of an epithelial layer on the wound surface. The final mucosal plate area 3 times exceeded the initial micrografts area. This initial clinical experience of using oral mucosa micrografts shows the new possibility of this technology in reconstructive urethral surgery, especially in patients with complex urethral strictures.
For citations:
Shibaev A.N., Pavlova Yu.V., Bazaev V.V., Podoinitsyn A.A., Sultanov D.I., Shinkarev A.D., Suleimanov R.S. A new approach to use of oral mucosa in reconstructive urethral surgery: micrografts. Urology Herald. 2022;10(1):70-83. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-70-83
Introduction
Treatment of patients with urethral strictures is an urgent problem of reconstructive urology [1][2][3]. The introduction into the widespread practice of oral mucosal transplants to replace urethral defects has significantly improved the results of treatment and the quality of life of this category of patients [4]. Accessibility, good adaptation to a humid environment, and manipulation characteristics, as well as low morbidity of donor sites, have now made the oral mucosa the preferred graft for urethral reconstruction [5][6][7][8]. In this regard, the approaches to the reconstruction of extended, multiple, and recurrent urethral strictures have changed significantly, significantly reducing the number of two-stage plastic surgeries [6][8].
However, in the practice of any urethral surgeon, there are patients with so-called “complex strictures”, when the lack of sufficiently accessible, healthy, and reliable local tissues for urethral reconstruction makes it impossible to perform one-stage surgical treatment [9][10]. This is usually observed in patients with multiple unsuccessful reconstructions in the anamnesis, especially after correction of hypospadias and with lichen sclerosis. The use of an oral mucosal graft as a material to create a urethral pad also dominates other techniques. However, planned two-stage surgeries often turn into multi-stage ones with an endless series of revision or corrective operations. According to the literature data, from 26 to 50% of patients with ‘complex strictures’ require additional corrective steps [11][12]. The most common cause of this is a contracture of the oral mucosa graft (more than 22%) [13].
After repeated surgery interventions, the possibilities of reusing autologous grafts (flaps) are often limited. This forces reconstructive surgeons to look for new sources of plastic material (various auto-, allo-, and xenografts, synthetic materials). Recently, in such situations, they have been increasingly trying to use various tissue-engineering structures [14][15]. However, the effectiveness of using these materials in reconstructive surgery of the urethra at this stage leaves much to be desired. In addition, currently, the use of tissue-engineering structures using cellular technologies in clinical practice is legally limited [16], but the main factors hindering the development of this direction and its introduction into clinical practice are the complexity and high cost of technologies [14].
Meek et al. proposed an alternative technique, who in 1958 introduced the theory of marginal tissue regeneration, demonstrating the possibility of rapid healing of extensive skin lesions by using a large number of skin microtransplants (micrografts) with a size of less than 1–2 mm2, a significantly smaller total area compared to the area of the wound [17]. Currently, micrografts are successfully used in combustiology, dermatology, dentistry, orthopedics, and treatment of chronic wounds of various etiologies [18][19][20][21][22][23][24]. In reconstructive urology, this approach to the replacement of defects of the lower urinary tract has been tested only in an experiment [25][26][27][28].
The study aimed to evalute the possibility of using oral mucosal micrografts to form the urethral pad in multi-stage treatment of patients with extended urethral strictures.
Methods and materials
The study, approved by the local independent ethics Committee of the M.F. Vladimirsky State Medical University (Protocol No. 8 of June 13, 2019), consisted of two parts – experimental and clinical ones. In the first part of the study, the possibility of oral mucosa micrograft engraftment and the formation of an epithelial site during heterotopic transplantation was evaluated in an experimental model.
The experimental part of the study. The experiment was carried out in accordance with the ethical standards for the treatment of animals adopted by the European Convention for the Protection of Vertebrates Used for Research and Other Scientific Purposes, the Federation of European Associations for the Science of Laboratory Animals, and the International Council for the Science of Laboratory Animals. Twenty-two male Wistar rats weighing 300–400 g were used (the nursery of Scientific Center for Biomedical Technologies, Federal Medical and Biological Agency, Russian Federaion). The animals were kept in vivarium conditions on a free food regime, included in the experiment after acclimatization for a period of 14 days. After intravenous sedation with a solution of tiletamine and zolazepam – Zoletil® 100 (Virbac S.A., Carros, France) at a dose of 20 mg/kg with the addition of xylazine – Xyla® (Interchemie werken “De Adelaar” BV, Castenray, The Netherlands) at a dose of 4 mg/kg under aseptic conditions, a full-layer skin wound with a diameter of 15 mm was performed on the withers of the animals; the bottom of the wound was the fascia of M. carnosus. To maintain a moist environment, limit wound contraction and healing by the skin epithelium, a wound chamber was used according to the method described by Nuutila et al. [29]. The cameras were made of biocompatible photopolymer Med 610 (Stratasys Ltd., Rehovot, Israel) by 3D printing («ITK-Endoprint» LLC, Moscow, Russia). The inner diameter of the chamber was 12 mm. The camera was fixed to the bottom of the wound and the edges of the skin with nodular Prolene 2/0 sutures (Ethicon Inc., Johnson & Johnson Company, Cincinnati, OH, USA) (Fig. 1).
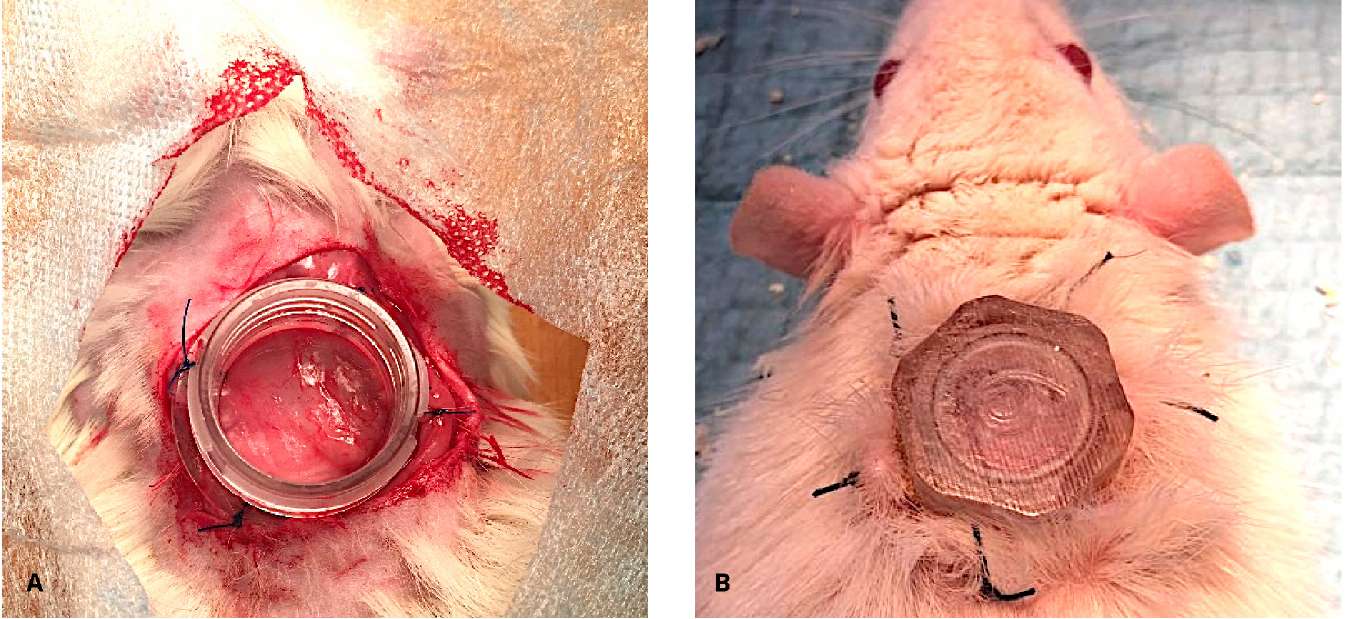
Figure 1. Wound chamber is placed transcutaneously on the dorsum of a rat (A, B)
After that, the mucosa in the oral cavity was taken. The oral cavity of the rat was treated with an aqueous solution of chlorhexidine. After hydrotreating with a 0.25% procaine solution – Novocaine («Grotex» JSC, St. Petersburg, Russian Federation), a section of the oral mucosa (6 mm in diameter) was taken with a Dermal Punch perforator (Miltex Inc., York, PA, USA) (the ratio between the graft area and the area inside the wound chamber was 1:4). The wound in the mouth was not sutured (Fig. 2).
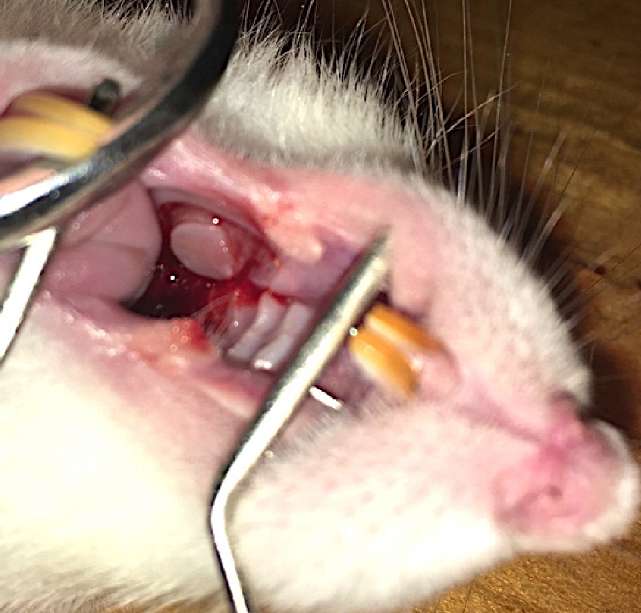
Figure 2. Oral mucosa graft harvesting
The graft was washed in an antibiotic solution containing Gentamicin 100 mg/l («Dalkhimpharm» JSC, Khabarovsk, Russian Federation) and Vancomycin 1000 mg/l («Pharm-Synthesis» JSC, St. Petersburg, Russian Federation), cleaned of the underlying fat, and mechanically crushed in 1 ml of the antibiotic solution to fragments < 1–2 mm in a sterile Petri dish (Fig. 3). After grinding, excess solutions were removed. The micrografts obtained were divided into two equal parts and mixed in different test tubes with the components of Cryophyte medical surgical glue fibrin-thrombin («Plasma-FTC» LLC, Zelenograd, Moscow, Russian Federation). Then, the glue components with suspended micrografts were alternately introduced into the wound (Fig. 4).

Figure 3. Oral mucosa micrografts
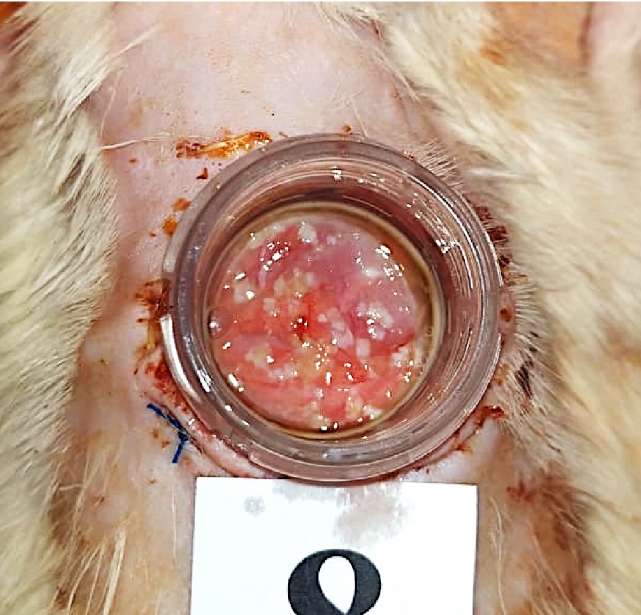
Figure 4. Oral mucosa micrografts with fibrin-thrombin glue in the wound
In the postoperative period, the animals underwent antibacterial therapy – a 10% solution of Enrofloxocin (ALPOVET Ltd., Surgis) 1 mg/kg/m for 7 days. Bandages under I/m sedation were performed 1 time in 3 days. At each dressing, a visual assessment of the wound was performed – the general condition, the amount and nature of the exudate, the state of micrografts. The wound chamber was kept much time as possible. With the eruption of more than half of the skin sutures, the wound chamber was removed. After the removal of the ring (and then on each dressing), the size of the wound was fixed with a ruler. On days 15 and 30, a punch biopsy was performed from the wound sites. The animals were removed from the experiment on day 45 due to excessive anesthesia drug administration. The area of the epithelized site was measured. The entire wound surface with the adjacent skin was excised in a single block. Tissue samples were fixed in a 10% neutral buffer solution of formalin; then serial sections were prepared according to the standard protocol. Five microns of sections were stained with hematoxylin and eosin. Histological examination assessed the degree of inflammatory reaction, the formation of the vascular network, the formation of granulation tissue, the engraftment of microphages, the area of epithelial growth, and its maturity. Histological images were obtained using the Leica Application Suite software for the Leica OFC450C light microscope (Leica Microsystems GmbH, Wetzlar, Germany).
The clinical part of the study. In the clinical part of the study, after signing informed consent, four patients with extended urethral strictures underwent reconstructive operations with the formation of the urethral pad and the use of micrografts of the oral mucosa.
In all the men patients, strictures were localized in the penile region and were recurrent. The average length of strictures was 7.5 ± 1.2 cm. In one of the patients, the penile urethra was stenosed all the way to the meatus. Three of the four had areas of obliteration of the lumen, one had a pronounced narrowing (< 2 mm) (Fig. 5).
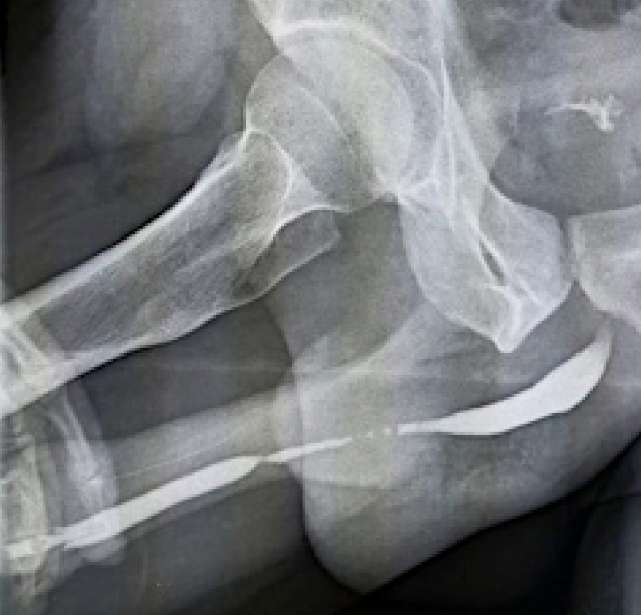
Figure 5. Retrograde urethrography: penile urethral stricture
At the time of the beginning of treatment, all patients had no independent urination; the duration of stay of cystostomy was from 6 to 12 months. When assessing the quality of life using the EQ-5D questionnaire (EuroQol questionnaire), all patients showed a marked decrease in indicators to 0.64 ± 0.05 (the maximum value of this indicator is 1). Evaluation of symptoms using the PROM-USS questionnaire (Patient-Reported Outcome Measure for Urethral Stricture Surgery questionnaire) before surgery was not performed due to the absence of self-urination.
In all patients, at the first stage of treatment, a urethral pad was formed using micrografts of the oral mucosa based on the methodology developed in the experimental part of the study. Under general anesthesia, the penile part of the urethra was isolated throughout; the lumen of the urethra was opened to an unchanged wall in both directions. After revision and excision of the altered urethra with a sclerosed spongy body, external urethral openings (proximal and distal urethral segments) were formed. The skin of the penis with Buck’s fascia was fixed, retreating 1.5–2 cm from the midline on each side, thus forming the bed boundaries for the transplantation of micrografts of the oral mucosa. The graft of the oral mucosa was taken according to the standard procedure. The calculation of its area was carried out based on measurements of the formed bed (in a ratio of 1:4). The further preparation of the micrografts and their implantation were carried out according to the procedure described above (Fig. 6).
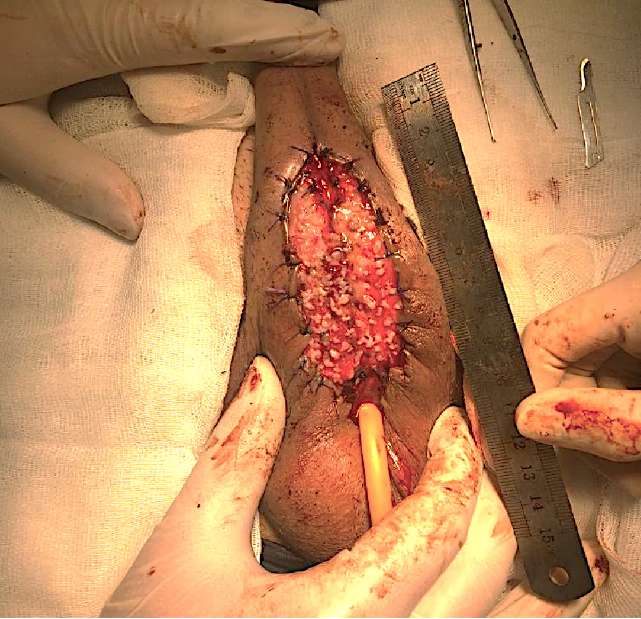
Figure 6. Staged urethroplasty: stage 1 – urethral plate formation
In the postoperative period, the wound surface was kept moist. Six months after the assessment of the site condition, the second stage of urethral plastic surgery was performed – tubularization stage. At the same time, a punch biopsy of the urethral pad was performed with a Dermal Punch perforator (Miltex Inc., York, PA, USA) with a diameter of 1 mm, followed by histological examination. During urethral tubularization, if the width of the urethral pad was less than 2.5 cm, an additional augmenting ventral insert was made from a graft of the oral mucosa using the onlay technique. The results of treatment were evaluated every 3 months (functional magnetic resonance imaging, bladder ultrasound with determination of residual urine, urethrography, evaluation of symptoms using the PROM-USS questionnaire) after the final stage of surgical treatment. The median follow-up was 9 (3–18) months.
Statistical analysis. Statistical data processing was carried out using the IBM SPSS Statistica 26 software package (StatSoft Inc., IBM SPSS Corp., Tulsa, OK, USA). The non-parametric Mann-Whitney criterion was used to identify differences in the values of the indicators. While testing hypotheses, the significance level as p < 0.05 was used.
Results
During the experimental part, no single animal was excluded from the study earlier than planned. In most of the animals (18 of 22), by day 21 of the experiment, the sutures that fixed the wound chamber had cut through, and therefore the chamber was removed. The observation of these animals was continued according to the protocol for up to 45 days.
On day 15, 16 out of 22 (72.7%) rats had focal growths of the multilayered non-keratinized epithelium (Fig. 7A). The actively granulating surface of the wound is covered with many rounded epithelization foci from micrografts that do not merge into a single epithelial layer (Fig. 7B).
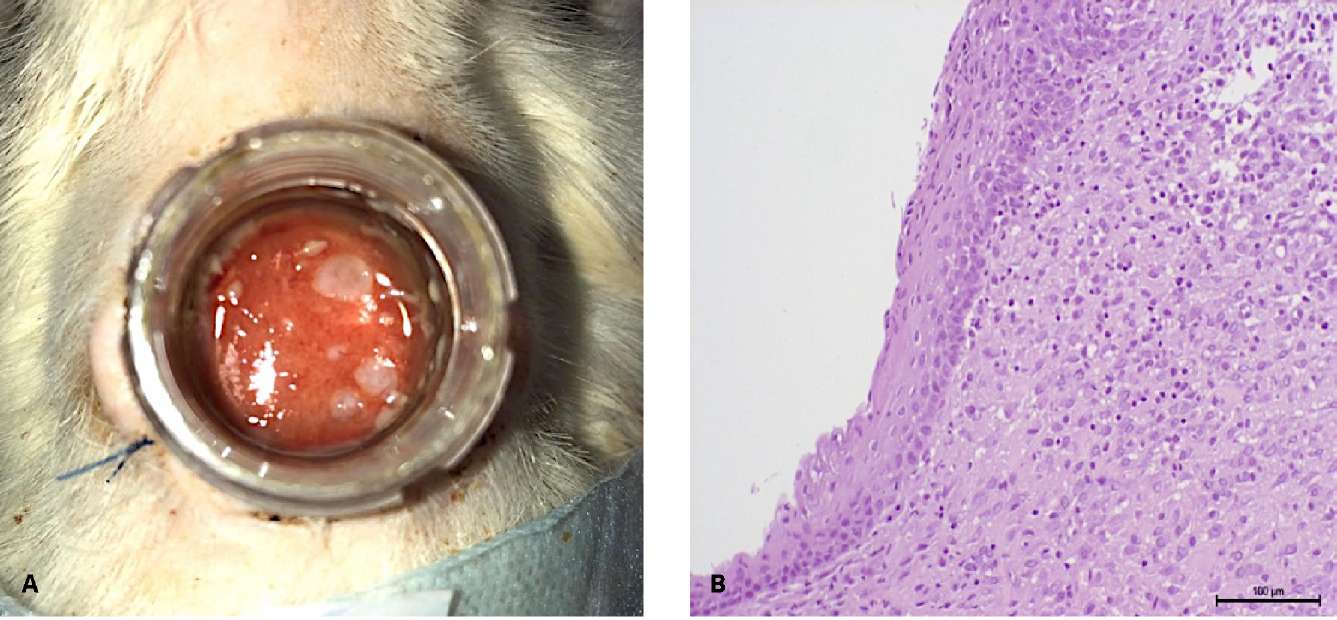
Figure 7. Follow-up day 15: A — type of wound: epithelialization sites;
B — focal growths non-keratinized stratified squamous epithelium
(hematoxylin-eosin, magn. ×200)
In part of the animals (n = 5), from day 7, the wound surface was covered with fibrinous-purulent overlays, under which epithelization was also observed with a non-keratinized multilayer epithelium.
On day 30, epithelization of the wound with a flat multilayer non-keratinized epithelium (epithelium of the oral cavity) took place in 15 of 22 rats (68.2%) (Fig. 8). At the same time, in 2 (9.1%) animals, the formation of epithelial microcysts was observed in the thickness of the wound granulation tissue, lined with the same flat multilayer epithelium, and containing keratous masses.
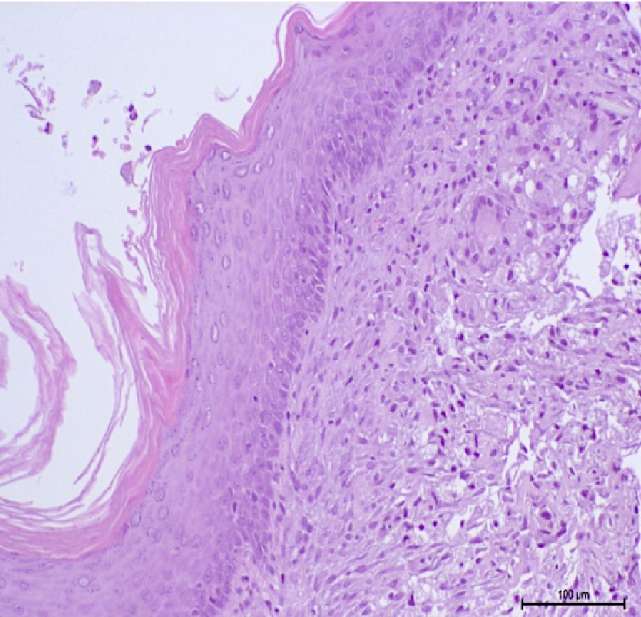
Figure 8. Follow-up day 30: formation of the non-keratinized stratified squamous epithelial layer
under a fibrin coating (hematoxylin-eosin, magn. ×200)
On day 45, complete wound healing with epithelization was observed due to the oral cavity epithelium with focal hyperparakeratosis. However, the basal plate of the epithelium was not reliably determined at this stage. The area of the resulting site from the epithelium of the oral cavity was 78 = 12 m2, which is on average 3 times larger than the area of the original graft (Fig. 9).
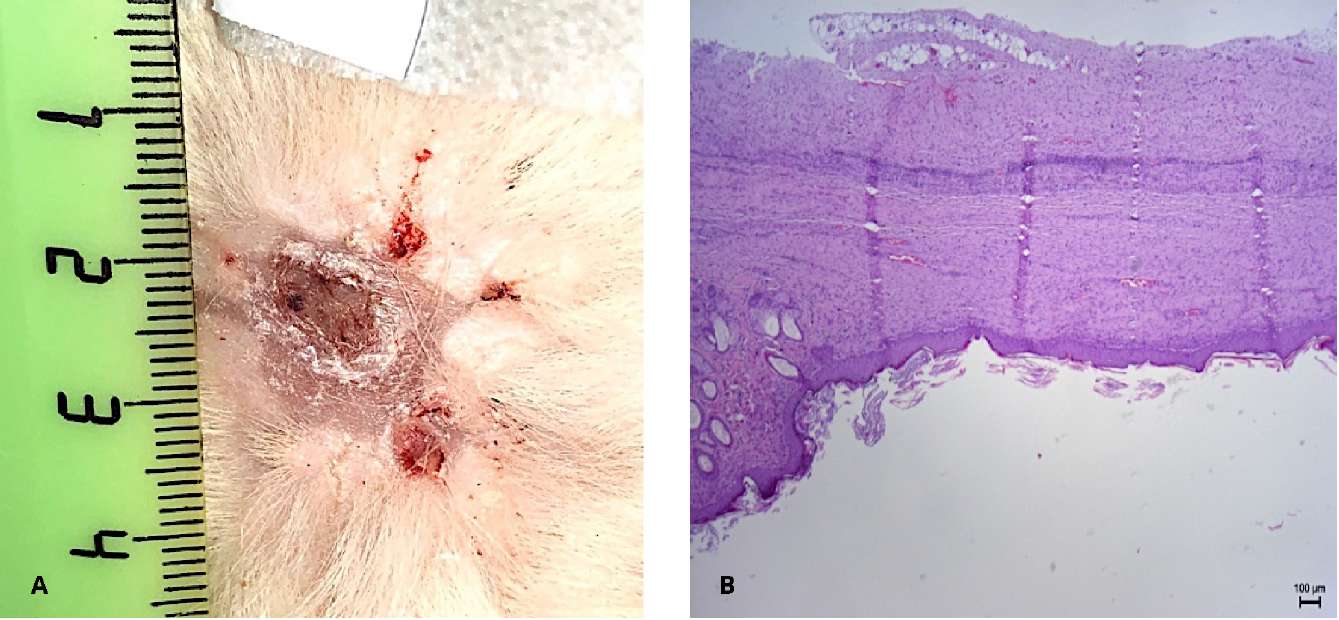
Figure 9. Follow-up day 45: A — wound epithelialization by oral mucosa.
B — stratified non-keratinized squamous epithelium (hematoxylin-eosin, magn. ×100)
The results of the clinical part of the study were obtained by dynamic observation of patients after the first and second stages of surgery. By the fourth to sixth month, all men had formed a urethral area without pronounced scarring (Fig. 10). According to the histological conclusion, a multilayer flat non-keratinized epithelium with the phenomenon of hyperparakeratosis and a formed own plate was determined.
All patients underwent urethral tubularization. In two patients, due to difficulties in mobilizing the edges of the site for tubularization, an additional 1 cm wide oral mucosa graft was used, fixed to the edges of the site ventrally. Additionally, the seam line was covered with a tunica Dartos flap.
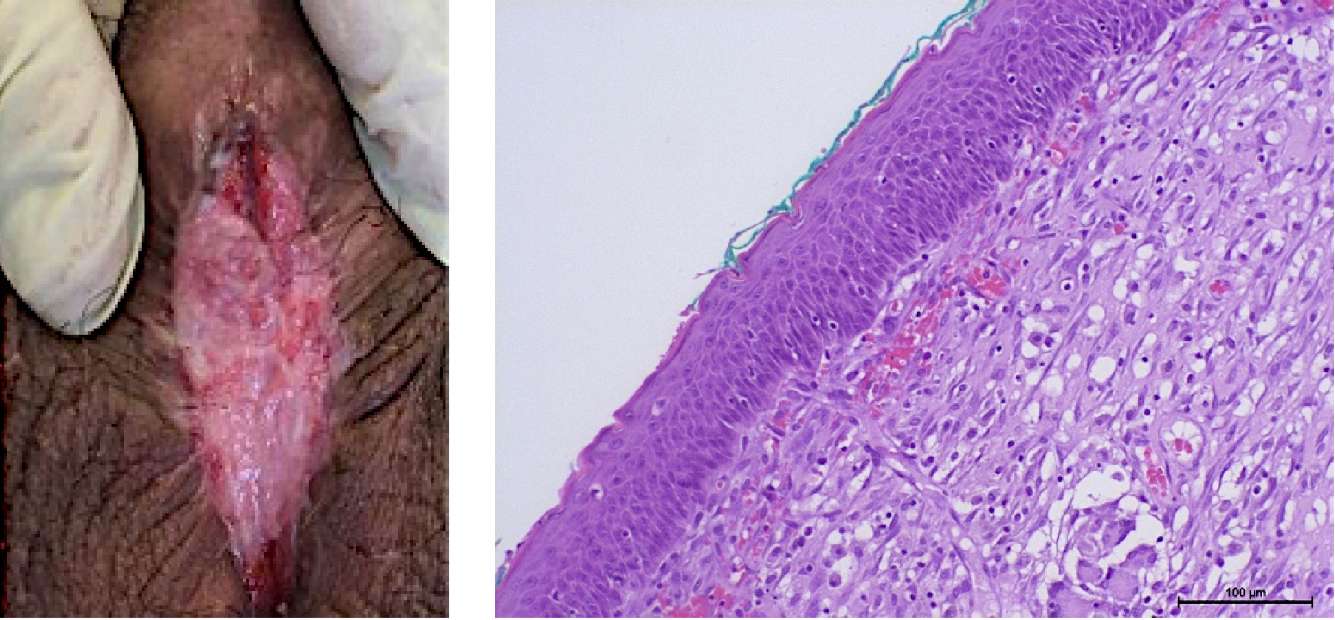
Figure 10. Patient T. Six months after stage 1 of urethroplasty: A — urethral plate;
B — non-keratinized stratified squamous epithelium (hematoxylin-eosin, magn. ×200)
After catheter removal (21 days after surgery), normal independent urination was restored in all men. The maximum urination rate was 22.7 ± 4.2 ml/s (9 months after surgery, n = 3) and then did not change significantly during the entire follow-up period. The average volume of residual urine after surgery was 34.8 ± 2.2 ml; with further observation, this indicator varied from 26.5 ± 5.3 ml to 43.8 ± 4.2 ml (p > 0.05 between points). According to the urethrographic data, an adequate urethral lumen remained in all patients (Fig. 11).
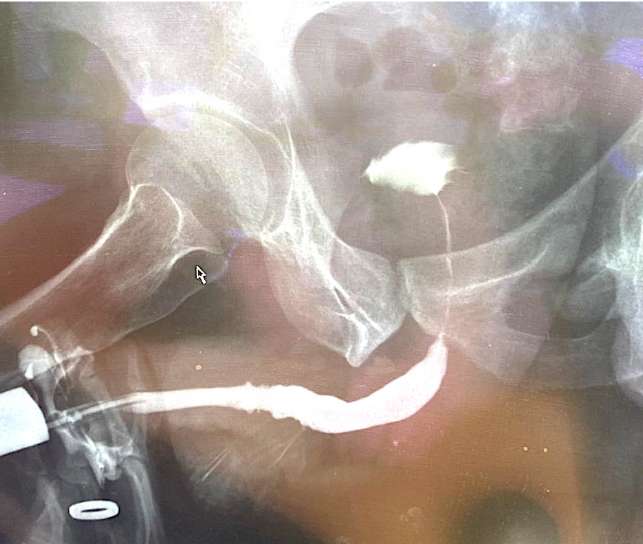
Figure 11. Retrograde urethrography 6 months after neourethra tubularization
While analyzing the results of the survey of men with the PROM-USS questionnaire 6 months after surgical treatment, the total score for assessing symptoms was 7.4 ± 1.2. While assessing the quality of life with the EQ-5D questionnaire, a significant increase in these indicators was revealed from 0.64 ± 0.0 to 0.79 ± 0.2 (p < 0.05). All patients noted high satisfaction with the results of treatment.
Discussion
The problem of shortage of plastic material is not new for reconstructive surgeons of various specialties. One of the solutions to this problem is the use of micrografts, the use of which significantly reduces the need for donor material and reduces the time of wound epithelization [17]. In reconstructive urology, several experimental studies have been published using micrografts (bladder mucosa, skin, and oral mucosa) to restore the lower urinary tract [25][26][27][28]. Even though the transitional cell epithelium of the bladder is related to the urethral epithelium, receiving bladder transplants is associated with significant morbidity. At the same time, oral mucosal transplants have been used in reconstructive urology for a long time and with great success due to good accessibility and relatively low morbidity of the fence [5][8][30]. Therefore, in this work, the research team also preferred to use micrografts of the oral mucosa. Considering their small size, their fixation to the bed was carried out using fibrin-thrombin surgical glue.
In this study, for the first time, the possibility of forming an epithelial site from micrografts lined with the epithelium of the oral mucosa exceeding by 3–4 times the area of the tissue used for this purpose was shown. The use of a wound chamber made it possible to significantly reduce the effect of wound contraction (especially in the first two weeks after transplantation) and subsequently use experimental results in the development of new technology for the treatment of patients with extended urethral strictures. In the clinical part of the study, four cases of the first in clinical practice of the use of micrografts of the oral mucosa with fibrin-thrombin glue for the formation of the urethral pad in the multi-stage treatment of such patients are presented. Very encouraging results have been obtained in this pilot series. In the sixth month, a mature urethral site is formed without pronounced scar deformities and sufficient dimensions for subsequent tubularization of the neourethra. Currently, it is too early to talk about the effectiveness of the presented technique due to the small sample of patients and short follow-up periods. Among its possible advantages, it is necessary to note the careful use of the oral mucosa during reconstructive operations on the urethra, a reduction in the number of treatment stages due to the absence of pronounced scarring of the epithelial site and neourethra. All this should lead to greater satisfaction for the patient and the doctor with the results of treatment.
Currently, various tissue engineering structures are being actively developed to replace the scarred urethra. As a rule, they all consist of one matrix or another and keratinocytes developed from the primary culture of epithelial cells in the patient's oral cavity in vitro (by themselves or in the form of epithelial spheroids) [31]. The proposed technology using micrografts is essentially an analog of such a tissue-engineered structure created without the use of cellular technologies and, therefore, without their inherent disadvantages (the high cost of reagents and equipment for in vitro cultivation, legislative restrictions on clinical use, etc.).
Thus, a new approach to the use of the oral mucosa in reconstructive surgery of the urethra by means of using micrografts is promising for clinical study, is characterized by relative simplicity and the possibility of implementation in a standard surgery room.
Conclusion
According to the data from the experimental stage of the studies, oral mucosal micrografts take root adequately during heterotopic transplantation in a wet wound (72.7%). By the day 21 after transplantation, they can create a single epithelial layer on the surface of the wound, the area exceeding the total area of the micrografts by 3 – 4 times. However, the basal plate of the epithelium has not yet been formed by this time, which theoretically limits the possibility of tubularization at these terms.
The first clinical experience of using oral mucosal micrografts shows the possibilities of using this technology in reconstructive surgery of the urethra, especially with 'complex strictures' of the urethra and with a shortage of plastic material. The results of this particular study show that in two out of four patients included in the study, this technique allowed the formation of a urethral pad of the required size without pronounced scar deformities, sufficient for the subsequent formation of the urethra. At the same time, it is possible to use significantly smaller grafts of the oral mucosa in the total area. It is too early to draw final conclusions about the effectiveness of this approach. It is necessary to evaluate it on sufficient material, with long follow-up periods, and in various clinical situations.
References
1. Kogan M.I., Krasulin V.V., Mitusov V.V., Shangichev V.A., Ametov R.E., Baranov S.V. Effectiveness of surgical treatment of extended and Subtotal urethral strictures in men. Medical Bulletin of Bashkortostan. 2013;8(2):95-97. (In Russ.). eLIBRARY ID: 20170231.
2. Kotov S.V. Results of multi-stage (replacement) urethroplasty. Experimental and Clinical Urology. 2015;(4):60-66. (In Russ.). eLIBRARY ID: 25659705.
3. Kulkarni S, Kulkarni J, Surana S, Joshi PM. Management of Panurethral Stricture. Urol Clin North Am. 2017;44(1):67-75. DOI: 10.1016/j.ucl.2016.08.011. Erratum in: Urol Clin North Am. 2017;44(2):xix. PMID: 27908373.
4. Dubey D, Vijjan V, Kapoor R, Srivastava A, Mandhani A, Kumar A, Ansari MS. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol. 2007;178(6):2466-9. DOI: 10.1016/j.juro.2007.08.010.
5. Barbagli G, Fabri F, Romano G, Michele D, Lazerri M. Evaluation of early, late complications and patient satisfaction in 300 patients who underwent oral graft harvesting from a single cheek using a standardised technique in a referral center experience. J Urol suppl. 2009;181:14. DOI: 10.1016/S0022-5347(09)60052-5.
6. Wessells H, Angermeier K W, Elliott S, Gonzalez CM, Kodama R, Peterson AC, Reston J, Rourke K, Stoffel JT, Vanni AJ, Voelzke BB, Zhao L, Santucci RA. Male urethral stricture: American urological association guideline. J Urol. 2017;197(1):182-190. DOI: 10.1016/j.juro.2016.07.087.
7. El-Kasaby AW, Fath-Alla M, Noweir AM, el-Halaby MR, Zakaria W, el-Beialy MH. The use of buccal mucosa patch graft in the management of anterior urethral strictures. J Urol. 1993;149:276-278. DOI: 10.1016/s0022-5347(17)36054-8.
8. Chapple C, Andrich D, Atala A, Barbagli G, Cavalcanti A, Kulkarni S, Mangera A, Nakajima Y. SIU/ICUD Consultation on Urethral Strictures: The management of anterior urethral stricture disease using substitution urethroplasty. Urology. 2014;83(3 Suppl):S31-47. DOI: 10.1016/j.urology.2013.09.012.
9. Barbagli G, De Angelis M, Palminteri E, Lazzeri M. Failed hypospadias repair presenting in adults. Eur Urol. 2006;49:887-894. DOI: 10.1016/j.eururo.2006.01.027.
10. Joshi, P M, Barbagli G, Batra V, Surana S, Hamouda A, Sansalone S, Costi D, Lazzeri M, Hunter C, Desai D J, Castiglone F, Kulkarni S B. A novel composite two-stage urethroplasty for complex penile strictures: A multicenter experience. Indian J Urol. 2017;33(2):155-158. DOI: 10.4103/0970-1591.203426.
11. Kozinn SI, Harty NJ, Zinman L, Buckley JC. Management of complex anterior urethral strictures with multistage buccal mucosa graft reconstruction. Urology. 2013;82(3):718-22. DOI: 10.1016/j.urology.2013.03.081.
12. Andrich DE , Greenwell TJ, Mundy AR. The problems of penile urethroplasty with particular reference to 2-stage reconstructions. J Urol. 2003;170(1):87-9. DOI: 10.1097/01.ju.0000069721.20193.fd.
13. Myers JB, McAninch JW, Erickson BA, Breyer BN. Treatment of adults with complications from previous hypospadias surgery. J Urol. 2012;188 (2):459-463. DOI: 10.1016/j.juro.2012.04.007.
14. Rashidbenam Z, Jasman MH, Hafez P, Tan GH, Goh EH, Fam XI, Kong Ho CC, Zainuddin Z, Rajan R, Nor FM, Shuhaili MA, Kosai NR, Imran F H, Ng MH. Overview of Urethral Reconstruction by Tissue Engineering: Current Strategies, Clinical Status and Future Direction. Tissue Eng Regen Med. 2019;16(4):365-384. DOI: 10.1007/s13770-019-00193-z.
15. Osman NI, Hillary C, Bullock AJ, MacNeil S, Chapple CR. Tissue engineered buccal mucosa for urethroplasty: progress and future directions. Adv Drug Deliv Rev. 2015 Mar;82-83:69-76. DOI: 10.1016/j.addr.2014.10.006.
16. Federal'nyj zakon Rossijskoj Federacii ot 23 ijunja 2016 g. №180-F3 "O biomedicinskih kletochnyh produktah" (s izmenenijami i dopolnenijami). (In Russ.). Accessed Feb 26, 2022. https://base.garant.ru/71427992/
17. Meek CP. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 1958;96:557-558. DOI: 10.1016/0002-9610(58)90975-9.
18. Hu G, Zhang P, Chen Y, Yuan Z, Song H. Efficacy of two-stage Meek micrografting in patients with severe burns. J Burn Care Res. 2021;irab241. DOI: 10.1093/jbcr/irab241.
19. Hackl F, Bergmann J, S. Granter S, Koyama T, Kiwanuka E, Zuhaili B, Pomahac B, Caterson E J, Junker J, Eriksson E. Epidermal Regeneration by Micrograft Transplantation with Immediate 100-Fold Expansion. Plastic and Reconstructive Surgery. 2012;129(3):443e-452e. DOI: 10.1097/PRS.0b013e318241289c.
20. Wang CH, Lin YJ, Hu S, Huang YL, Chung WH, Ng CY. Efficacy and safety of automated epidermal micrograft in patients with stable segmental and nonsegmental vitiligo. J Cosmet Dermatol. 2021. DOI: 10.1111/jocd.14548.
21. Pothula SR, Jayanth BS. Hair Transplantation. Oral and Maxillofacial Surgery for the Clinician. 2021:707-730. DOI: 10.1007/978-981-15-1346-6_34.
22. Marcarelli M, Zappia M, Rissolio L, Baroni C, Astarita C, Trovato L, Graziano A. Cartilage Micrografts as a Novel Non-Invasive and Non-Arthroscopic Autograft Procedure for Knee Chondropathy: Three-Year Follow-Up Study. J Clin Med. 2021;10(2):322. DOI: 10.3390/jcm10020322.
23. Jimi S, Takagi S, De Francesco F, Miyazaki M, Saparov A. Acceleration of Skin Wound-Healing Reactions by Autologous Micrograft Tissue Suspension. Medicina (Kaunas). 2020;56(7):321. DOI: 10.3390/medicina56070321.
24. Miranda R, Farina E, Farina MA. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J Wound Care. 2018;27(2):60-65. DOI: 10.12968/jowc.2018.27.2.60.
25. Ajalloueian F, Zeiai S, Rojas R, Fossum M, Hilborn J. One-stage tissue engineering of bladder wall patches for an easy-to-use approach at the surgical table. Tissue Eng Part C Methods. 2013;19(9):688-96. DOI: 10.1089/ten.TEC.2012.0633.
26. Nikolavsky D, Manwaring J, Bratslavsky G, Caza T, Landas S, Hryniewicz-Jankowska A, Kotula L. Novel Concept and Method of Endoscopic Urethral Stricture Treatment Using Liquid Buccal Mucosal Graft. J Urol. 2016;196(6):1788-1795. DOI: 10.1016/j.juro.2016.05.028.
27. Engberg GR, Chamorro CI, Nordenskjöld A, Fossum M. Expansion of Submucosal Bladder Wall Tissue In Vitro and In Vivo. Biomed Res Int. 2016;2016:5415012. DOI: 10.1155/2016/5415012.
28. Chamorro CI, Zeiai S, Reinfeldt Engberg G, Fossum M. Minced Tissue in Compressed Collagen: A Cell-containing Biotransplant for Single-staged Reconstructive Repair. J Vis Exp. 2016;(108):53061. DOI: 10.3791/53061.
29. Nuutila K, Singh M, Kruse C, Philip J, Caterson EJ, Eriksson E. Titanium wound chambers for wound healing research. Wound Repair Regen. 2016;24(6):1097-1102. DOI: 10.1111/wrr.12472.
30. Lumen N, Campos-Juanatey F, Greenwell T, Martins FE, Osman NI, Riechardt S, Waterloos M, Barratt R, Chan G, Esperto F, Ploumidis A, Verla W, Dimitropoulos K. European Association of Urology Guidelines on Urethral Stricture Disease (Part 1): Management of Male Urethral Stricture Disease. Eur Urol. 2021;80(2):190-200. DOI: 10.1016/j.eururo.2021.05.022.
31. Zurina IM, Shpichka AI, Saburina IN, Kosheleva NV, Gorkun AA, Grebenik EA, Kuznetsova DS, Zhang D, Rochev YA, Butnaru DV, Zharikova TM, Istranova EV, Zhang Y, Istranov LP, Timashev PS. 2D/3D buccal epithelial cell self-assembling as a tool for cell phenotype maintenance and fabrication of multilayered epithelial linings in vitro. Biomed Mater. 2018;13(5):054104. DOI: 10.1088/1748-605X/aace1c.
About the Authors
A. N. ShibaevRussian Federation
Andrew N. Shibaev — M.D., Cand.Sc.(Med); Leading Researcher, Urology Division
Moscow
Yu. V. Pavlova
Russian Federation
Yulia V. Pavlova — M.D., Cand.Sc.(Med); Researcher, Urology Division
Moscow
V. V. Bazaev
Russian Federation
Vladimir V. Bazaev — M.D., Dr.Sc.(Med); Full Prof.; Leading Researcher, Urology Division
Moscow
A. A. Podoinitsyn
Russian Federation
Alexey A. Podoinitsin — M.D., Dr.Sc.(Med); Head, Urology Division
Moscow
D. I. Sultanov
Russian Federation
Dmitry I. Sultanov — Resident, Urology Division
Moscow
A. D. Shinkarev
Russian Federation
Artem D. Shinkarev — Cadet
St. Petersburg
R. S. Suleimanov
Russian Federation
Ruslan S. Suleymanov — M.D.; Urologist, Surgery Division
Tver
Review
For citations:
Shibaev A.N., Pavlova Yu.V., Bazaev V.V., Podoinitsyn A.A., Sultanov D.I., Shinkarev A.D., Suleimanov R.S. A new approach to use of oral mucosa in reconstructive urethral surgery: micrografts. Urology Herald. 2022;10(1):70-83. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-70-83













































