Scroll to:
The non-biological simulator with the ability to regulate the position of the kidney and bone landmarks: use for training puncture access in percutaneous nephrolithotripsy
https://doi.org/10.21886/2308-6424-2022-10-1-5-14
Abstract
Introduction. The non-biological simulators presented in the literature are far from the real human anatomy and are primarily aimed at developing the skill of the pyelocalyceal system (PCS) puncture without the possibility of imitating various intraoperative scenarios.
Purpose of the study. To describe the manufacturing and initial testing of the ultrasound-guided PCS puncture simulator with arbitrary placement of bone landmarks and a kidney model, along with the use of a retrograde view during PCS puncture.
Materials and methods. This study included training for 5 resident and 2 urologists. Each participant performed the puncture 5 times using an 18-gauge ultrasound-guided needle. A comparison was made between the number of attempts to form access, the duration of the puncture and its correctness (puncture into the small calyx through the papilla), as well as the correctness of determining the target calyx. The trajectory of the needle was retrogradely assessed using a semi-rigid ureteroscope, and the anatomical identification of the selected calyx was assessed using our mobile application.
Results. The total number of attempts was 49 and 14 among residents and urologists, respectively. The average duration of the puncture step was 25.2 and 12.0 seconds. In 9/25 cases, residents were able to correctly analyze visual ultrasound information to determine the target calyx. When a contrast agent was injected into the PCS after 63 punctures, no contrast leakage was found.
Conclusion. The proposed PCS puncture simulator allows to develop to develop all the necessary skills for cost-effective training of young urologists in the technique of percutaneous access.
Keywords
For citations:
Guliev B.G., Talyshinskiy A.E., Stetsik E.O., Agagyulov M.U. The non-biological simulator with the ability to regulate the position of the kidney and bone landmarks: use for training puncture access in percutaneous nephrolithotripsy. Urology Herald. 2022;10(1):5-14. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-5-14
Introduction
Percutaneous nephrolithotripsy (PCNL) is an endourological intervention, which is complicated in mastering and requires over 60 performed operations for significant experience [1]. Among its stages, accessing the pyelocalyceal system (PCS) is the most complicated one. It is characterized by a significant risk of associated complications (12.5–30.3%) [2]. This issue is acute in young specialists due to difficulties in the development of the skill of identifying tissues by their resistance to feedback during puncture and the association between tactile perception and visual information. The modern tendency for the solution of these problems is in the development of various simulators. Based on the technology of production, simulators are divided into the following groups: virtual reality (VR)-based, biological simulators made of animal tissues and organs, and non-biological ones that are made of various materials to imitate the real anatomy of the PCS and surrounding structures. Each group has its advantages and disadvantages. VR-based simulators do not require repeated manufacturing and imitate the picture of the C-arm, but their high cost is the main limiting factor for their wide implementation into medical institutions. Biological simulators provide sensations similar to those of a kidney puncture. In addition, due to the presence of several calices in the PCS of some animals, it is possible to train skills for various PCNL scenarios. However, their long-term storage and transportation are difficult because of organs decay. Non-biological simulators are the most available and variable in production, especially after the popularization of gelatin that can be reused multiple times. Still, the described simulators are far from real human anatomy and primarily target the training of the PCS puncture skill.
The study aimed to describe the manufacturing and initial testing of the ultrasound-guided PCS puncture simulator with an arbitrary placement of bone landmarks and a kidney model, along with the use of a retrograde view during PCS puncture training of residents.
Materials and methods
The computed tomography (CT) urography images of a patient with a stone (2.3 cm) in the left kidney pelvis were obtained after the patient signed informed consent. CT images were analyzed with the software DICOM Viewer (RadiAnt, Poznań, Poland). After 3D reconstruction of the excretory phase, the following structures were identified for further printing: 11th and 12th ipsilateral ribs, a fragment of an iliac wing, and the PCS of the affected kidney. Further, the left half of the lumbar area and the kidney parenchyma were identified for the formation of the respective molds. The data of the mentioned structures were saved in STL format and sent to a bioengineer for their preparation for 3D printing. Polylactide (PLA) was used as a material for the 3D printing of bone structures and body contours. The latter consists of the following parts: inferomedial, superolateral, and anterior walls made of metal to provide a fast gelatin conduction-type cooling down. The medial wall of the mold had a mounted plate for manual fixation of bone structures with magnets. The kidney mold is also made of PLA. A water-soluble printed kidney PCS was placed into the mold (Figure 1).
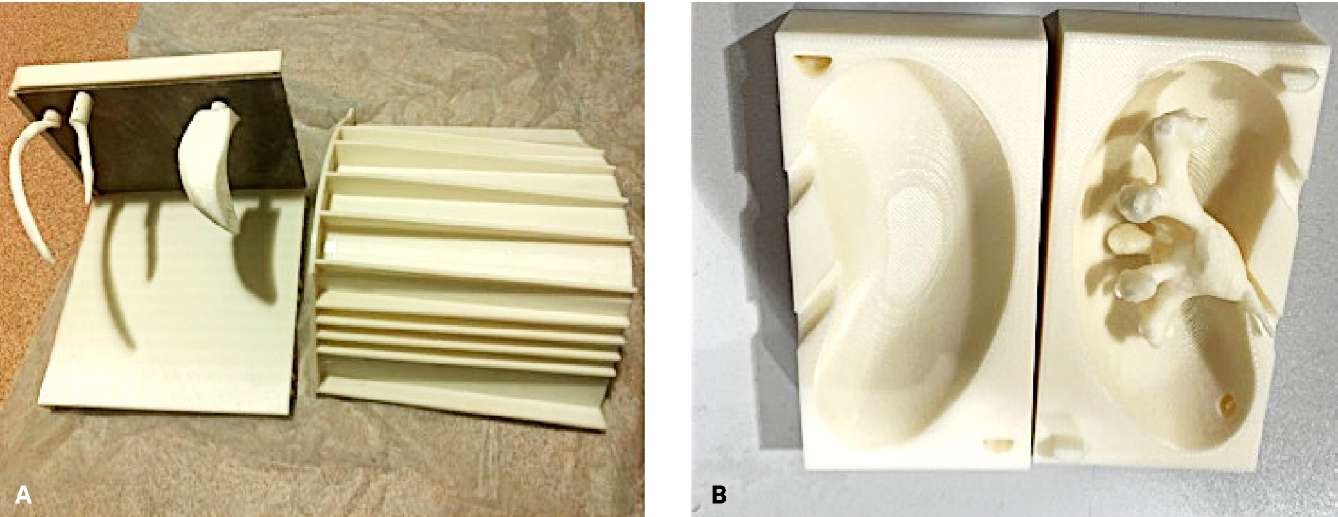
Figure 1. Printed molds for embedding the body with a metal plate on the medial wall
for fixing the ribs and a fragment of the iliac bone (A)
and for embedding the kidney with an in-located soluble pyelocalyceal system (B)
For better imitation of the kidney parenchyma properties, multicomponent silicone with 0–30 Shore was used. A Malecot 20 Ch catheter was used for a ureter.
Gelatin composition. Gelatin composition for filling the body mold directly depends on the echogenicity of the ultrasound (US) picture and sensations of resistance during the puncture. In this direction, the publication of Gadgieva et al. [3] attracted the attention of researchers. The mentioned publication aimed not only to describe the US-guided puncture simulator but also to choose the gelatin composition for its maximal durability with a possibility of material reutilization. The study results showed that the optimal ratio was glycerin (208 g):water (0 g):gelatin (42 g). This composition provides self-closure of the puncture ducts (if there is no critical damage), high speed of the US wave, and resistance at room temperature. In the present study, the following gelatin composition ratio was used: food gelatin (25%):glycerin (50%):water (25%), which was associated with a large volume of body contour mold (7 L).
Simulator assembly. CT images were used to identify the spaces between the 11th and 12th ribs and iliac wing, the distance between the mentioned bone landmarks and kidney, as well as the orientation of the kidney. Bone structures were placed on the medial wall of the body model considering the data obtained earlier. For stabilization of the kidney model obtained in space with regard to its poles and medial edge, magnet threads were attached, which allowed the authors to identify its orientation and depth of location in the simulator. When all components were placed, both body mold walls were connected and located vertically on a metal wall with further filling with gelatin. The filled mold was placed in a refrigerator for 24 hours to solidify (Figure 2).

Figure 2. The resulting kidney model with a Malecot catheter installed as a ureter
and with thread fixators to determine the depth and location of the kidney (A)
in the kidney puncture simulator after filling and solidifying of gelatin (B)
Simulator evaluation. The study included five first- and second-year residents (Group 1) and two urologists with experience of individual PCNL performance of > 60 surgeries (Group 2). Each participant made five puncture attempts (the authors counted the number of punctures in the small calix through the papilla) using an 18 gauge US-guided needle.
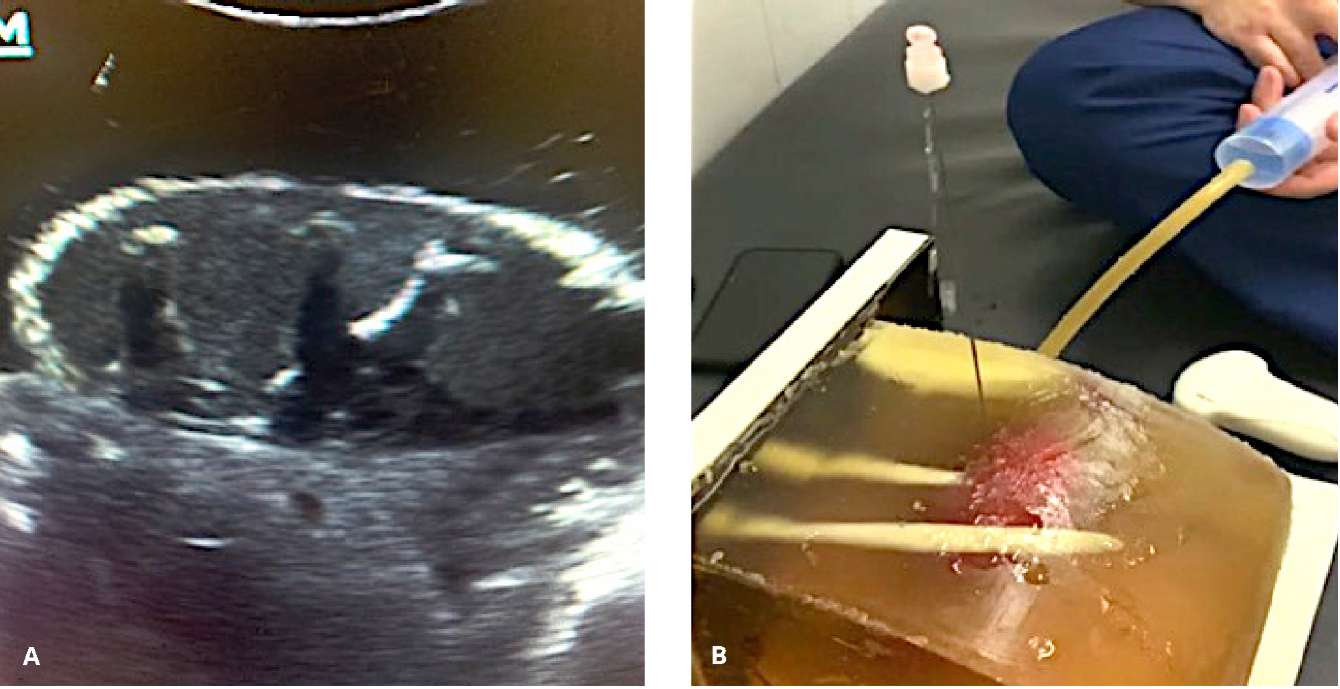
Figure 3. Ultrasound view of the modeled "parenchyma" and the pyelocalyceal system (A).
Retrograde infusion and fluid outflow after successful approbation puncture (B)
The authors made a comparison between the number of attempts to form access, the duration of the puncture, and its correctness, as well as the precision of determining the target calyx. Finally, after puncture, the participants identified the punctured calix according to the US image. For more precise identification, the needle trajectory was retrogradely evaluated using a semi-rigid ureteroscope. The anatomical determination of the target calix was performed with the mobile application InsKid (Inside Kidney) developed by the authors (Figure 4) [4].
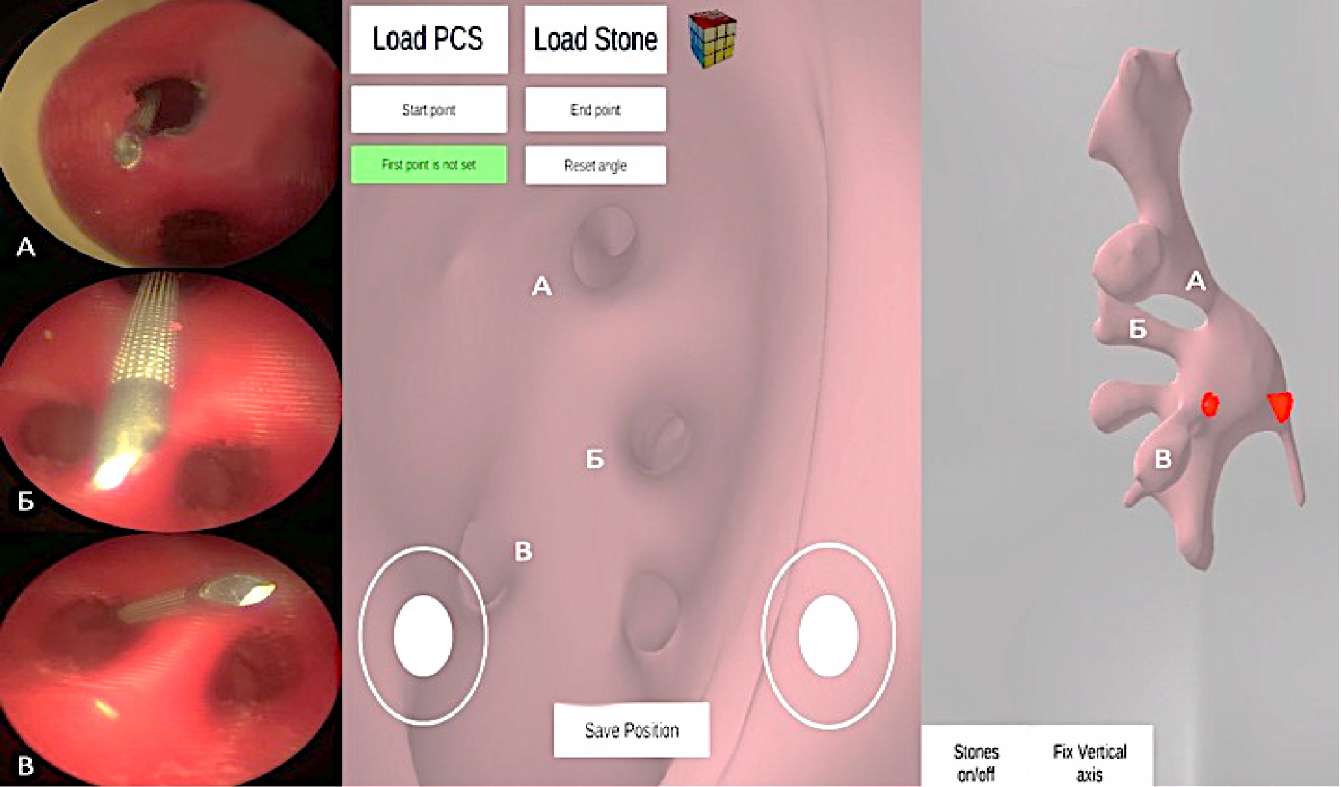
Figure 4. Retrograde evaluation of the needle puncture course
through the calyx of the upper (A), middle (B), and lower (C) groups.
Virtual endoscopy view (center and right figures)
obtained using a mobile application to accurately determine the punctured calyx
After the simulator approbation by the groups, a contrast agent was injected into the renal cavity to check its consistency and suitability for further application.
Statistical analysis. There was performed using IBMÒ SPSS Statistics 23 (SPSS: IBM Company, IBM SPSS Corp., Armonk, NY, USA). The variability of the data was described using the mean values with the specification of the maximal and minimal values. To evaluate continuous and discrete data, the Student test was used. Nominal data were assessed using the c-square test. The difference was significant at p < 0.05.
Results
Residents and urologists made a total of 49 and 14 puncture attempts, respectively, into the small calix through the papilla. The mean time of puncture stage was 25.2 and 12.0 seconds. Only in nine cases, residents could analyze properly the visual US images for correct calyx targeting, which shows the lack of experience in percutaneous manipulation (Table).
Table. Training performance among residents (Group 1) and urologists (Group 2)
|
Parameter |
Group 1 |
Group 2 |
p |
|
Attempts, n |
49 |
14 |
– |
|
Puncture time, sec |
25.2 (8.0–59.0) |
12.0 (7.0–21.0) |
< 0.05 |
|
Correct calyx targeting |
9/25 |
10/10 |
< 0.05 |
After 63 punctures (total number of punctures in both groups), a contrast agent was injected. No leakage was detected, which indicated the long-term durability of the proposed simulator.
Discussion
PCNL almost completely replaces open surgery in patients with nephrolithiasis [5]. However, there are some difficulties associated with it. First, surgery has several stages and the success of each stage depends on the adequate performance of the previous. Second, the surgeon should be sufficiently experienced in this technique. Schilling et al. [6] compared the results of this intervention carried out by surgeons with different experience and revealed a direct dependence between surgeon experience and the rate and significance of PCNL complications. Among the stages of PCNL, proper kidney access formation is critical because its correct performance minimizes intraoperative risks and simplifies nephroscopy and lithotripsy procedures. Furthermore, adequate access is necessary for some other manipulations (antegrade stenting, endopyelotomy, nephrostomy, and antegrade resection of upper urinary tract neoplasms). The access can be formed adequately by the surgeon if they have the appropriate skills to associate the visual picture and the tactile feedback of the resistance of the tissue.
Currently, young specialists have some difficulties in acquiring skills in PCNL performance. Not all urological units specialize in the surgical treatment of nephrolithiasis, which limits the number of patients enrolled for skills training without a decrease in the effectiveness of surgical interventions. Second, not all units have a C-arm, which negatively affects the training in this technique either.
The most promising training method is the application of simulators [7]. Ideally, the PCS puncture simulator should have the following properties: precise imitation of the kidney and its PCS, presence of support bone structures as landmarks (11th and 12th ipsilateral ribs and a fragment of the iliac bone), the possibility of variable location of the bones relative to each other and kidney model, presence of tactile sensations feedback of different tissue resistance, and the possibility of integration with US-investigation and a C-arm. There are publications on various approaches to the design of simulators that meet some of the mentioned requirements. There are also many publications on the design of simulators of several types: VR-simulators, biological simulators based on animal tissues and organs, and non-biological simulators applying various materials for the imitation of human tissues and the PCS.
Virtual reality simulators can be used without a real C arm and for puncture training in various scenarios, which is an advantage for qualitative preparation of surgeons for real-time practice with no potential harm to patients. An example of such a simulator is the PERC Mentor Suite (Simbionix Ltd., Beit Golan, Airport City, Israel) [8]. However, the high cost of the equipment and limited realism make it a less attractive alternative in practice.
Simulators based on animal tissues and organs primarily involve the application of pig kidneys because of the multi-calyx structure of their PCS. Strohmaier et al. [9] described the design of such a simulator that was based on the excision of the pig kidneys with the surrounding structures of the retroperitoneum. In addition to that, the kidney cavity was opened for the placement of a kidney stone sample in it to train all stages of PCNL with fluoroscopy. In addition, there are some more complicated methods of biological simulators described. Hammond et al. [10] placed a pig kidney with a stone into a chicken. The contrast agent was injected via a ureter catheter to the PCS for radioscopy visualization during a puncture tract expansion, and introduction of a tube shield. Besides, this model can be used for training the skills in nephroscopy, lithotripsy, and removal of stone fragments. The procedure can be repeated 3 times using one model. The description of biological models shows their advantages and disadvantages. The application of living tissues imitates the tissue resistance of the needle when making access on a human body. Still, such simulators require careful handling and storage, which explains their short-term application.
The application of non-biological simulators solves the issues that face the users of VR and biological simulators. They are simple in use, structurally variable, and provide long-term service within several months if stored adequately. However, their disadvantage lies in unrealistic human anatomy.
The authors believe that the study by Ali et al. [11] describes the most realistic body and kidney model using a 10 Shores silicon density. The density of the simulator components is close to real human tissue throughout the course of the puncture needle. Furthermore, the model contains bone landmarks such as the 11th and 12th ribs, thoracolumbar vertebrae, and a fragment of an iliac bone. The model was tested with fluoroscopic and US investigation methods, which confirmed its usefulness in the training of young specialists. However, silicone damage, the impossibility of its reuse, and correction of bone landmark positions limit the application of this simulator in planning each PCNL surgery and daily routine training of surgeons due to the necessity to buy more silicon and spend time on kidney preparation.
A simulator that is simpler in design was described by Aro et al. [12] who used the technology of 3D printing to create the mold with a contour of the lumbar human area for placing a soluble printed PCS model in it. The lumbar area model was filled with gelatin. After gelatin solidification, the kidney cavity was dissolved. The possibility of gelatin reuse made the simulator a cheap option for training and fast production. However, 3D printing of PCS with soluble polymers is a limiting factor that elongates the simulator preparation time. Apart from this, the simulator does not have bone landmarks and a separate channel into the renal cavity, which reduces the realistic properties of the simulator and does not provide the possibility of retrograde nephroscopy as an individual manipulation and for the evaluation of the percutaneous puncture.
A more available option is the formation of PCS from a disposable glove placed into a rectangular cavity filled with gelatin, which was described in the study by Septian et al. [13]. Besides, the authors placed a model of the 12th rib to improve the imitation of the puncture point planning. Still, the lack of qualitative imitation of real PCS anatomy and a rectangular shape of the simulator negatively affects the similarity of training and real surgery.
The described examples show that an increase in similarity to real human anatomy increases the cost and time required for the preparation of such simulators. A simpler approach to the imitation of kidney PCS results in a decrease in the real anatomic properties of the model.
To avoid such drawbacks, the authors of the present study proposed some solutions. First, the application of the described composition provides a significant volume of filler without affecting its reutilization. Second, the contour of the simulator shaped as a human lumbar area and the application of the 11th and 12th ipsilateral ribs and a fragment of an iliac bone with their manual positioning on the medial wall of the simulator provide a real anatomy model of a given patient. Moreover, these structures can be placed relative to each other and relative to the silicon kidney, which artificially changes the difficulty of such training and prepares the trained surgeons for various scenarios. The application of various density materials to fill the body model and make a kidney provides a urologist with reliable tactile feedback during puncture. Third, the application of pink silicon to make a kidney and a catheter of a sufficient diameter to imitate a ureter makes it possible to perform retrograde nephroscopy, which can be used for the control and evaluation of a puncture demonstrated in the present study. The described solutions provide not only the training of puncture skills, but also the following stages of PCNL imitation.
Despite the described advantages, this simulator has its drawbacks. The preparation of a silicon kidney is a limiting factor because, after irreversible changes, a new kidney needs to be made, which takes around 12 hours. The tissues between the skin and kidney are made of homogenous gelatin, which does not provide tactile feedback when the needle goes through the fascia and muscles. It is impossible to assess artery damage during training because the proposed simulator does not contain artificial vessels. Despite these drawbacks, the results of the study confirm the feasibility of the proposed simulator to train young specialists and to prepare experienced urologists for various PCNL scenarios.
Conclusion
The proposed and approbated method of preparation of a simulator for kidney puncture training allows the specialists to develop the required skills and provides an economic solution for training of the young specialists. The application of the retrograde puncture control and mobile application navigation during the identification of certain parts of the PCS highlights the mistakes made by the surgeon, which increases the quality of the training process.
References
1. Allen D, O'Brien T, Tiptaft R, Glass J. Defining the learning curve for percutaneous nephrolithotomy. J Endourol. 2005;19(3):279-82. DOI: 10.1089/end.2005.19.279.
2. Ganpule AP, Vijayakumar M, Malpani A, Desai MR. Percutaneous nephrolithotomy (PCNL) a critical review. Int J Surg. 2016;36(Pt D):660-664. DOI: 10.1016/j.ijsu.2016.11.028.
3. Gadzhiev N.K., Mishchenko A.A., Britov V.P., Khrenov A.M., Gorelov D.S., Obidnyak V.M., Grigoriev V.E., Semenyakin I.V., Petrov S.B. Creation of a training simulator model for practising puncture of the kidney calyceal system under ultrasound control. Vestnik Urologii. 2021;9(1):22-31. (In Russ.). DOI: 10.21886/2308-6424-2021-9-1-22-31.
4. Guliev B, Komyakov B, Talyshinskii A. Interior definition of the calyceal orientation suitable for percutaneous nephrolithotripsy via mobile software. Urolithiasis. 2021;49(5):443-449. DOI: 10.1007/s00240-021-01253-7.
5. Chen Y, Feng J, Duan H, Yue Y, Zhang C, Deng T, Zeng G. Percutaneous nephrolithotomy versus open surgery for surgical treatment of patients with staghorn stones: A systematic review and meta-analysis. PLoS One. 2019;14(1):e0206810. DOI: 10.1371/journal.pone.0206810.
6. Schilling D, Gakis G, Walcher U, Stenzl A, Nagele U. The learning curve in minimally invasive percutaneous nephrolitholapaxy: a 1-year retrospective evaluation of a novice and an expert. World J Urol. 2011;29(6):749-53. DOI: 10.1007/s00345-010-0553-3.
7. Jutzi S, Imkamp F, Kuczyk MA, Walcher U, Nagele U, Herrmann TR. New ex vivo organ model for percutaneous renal surgery using a laparoendoscopic training box: the sandwich model. World J Urol. 2014;32(3):783-9. DOI: 10.1007/s00345-013-1151-y.
8. Noureldin YA, Andonian S. Simulation for Percutaneous Renal Access: Where Are We? J Endourol. 2017;31(S1):S10-S19. DOI: 10.1089/end.2016.0587.
9. Strohmaier WL, Giese A. Improved ex vivo training model for percutaneous renal surgery. Urol Res. 2009;37(2):107-10. DOI: 10.1007/s00240-009-0180-x.
10. Hammond L, Ketchum J, Schwartz BF. A new approach to urology training: a laboratory model for percutaneous nephrolithotomy. J Urol. 2004;172(5 Pt 1):1950-2. DOI: 10.1097/01.ju.0000140279.15186.20.
11. Ali S, Sirota E, Ali H, Bezrukov E, Okhunov Z, Bukatov M, Letunovskiy A, Grygoriev N, Taratkin M, Vovdenko S, Afyouni A, Alyaev Y. Three-dimensionally printed non-biological simulator for percutaneous nephrolithotomy training. Scand J Urol. 2020;54(4):349-354. DOI: 10.1080/21681805.2020.1773529.
12. Aro T, Lim S, Petrisor D, Koo K, Matlaga B, Stoianovici D. Personalized Renal Collecting System Mockup for Procedural Training Under Ultrasound Guidance. J Endourol. 2020;34(5):619-623. DOI: 10.1089/end.2019.0735.
13. Septian R, Adi K. Validation of affordable and applicable kidney phantom model (aarm) for ultrasound-guided percutaneous nephrostomy simulation. Indones J Urol. 2020;27(1):26-33. DOI: 10.32421/juri.v27i1.515.
About the Authors
B. G. GulievRussian Federation
Bakhman G. Guliev — M.D., Dr. Sc. (Med), Full Prof.; Prof., Head, Urology Centre with Robot-assisted Surgery
St. Petersburg
A. E. Talyshinskiy
Russian Federation
Ali E. Talyshinskiy — Resident
St. Petersburg
E. O. Stetsik
Russian Federation
Evgeniy O. Stetsik — M.D., Urologist; Postgraduate student
St. Petersburg
M. U. Agagyulov
Russian Federation
Murad U. Agagyulov — M.D., Urologist; Postgraduate student
St. Petersburg
Review
For citations:
Guliev B.G., Talyshinskiy A.E., Stetsik E.O., Agagyulov M.U. The non-biological simulator with the ability to regulate the position of the kidney and bone landmarks: use for training puncture access in percutaneous nephrolithotripsy. Urology Herald. 2022;10(1):5-14. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-1-5-14













































