Scroll to:
Multimodal therapy for oligometastatic prostate cancer: results from a single-centre study
https://doi.org/10.21886/2308-6424-2021-9-4-70-86
Abstract
Introduction. In recent years, interest in the use of radical prostatectomy (RPE) as one of the components of a multimodal approach in patients with lymphogenous disseminated and metastatic prostate cancer (PCa) has grown significantly. At the same time, the dearth of large randomized trials does not make it possible to use this technique in wide clinical practice outside of clinical trials.
Purpose of the study. To evaluate the effectiveness of multimodal therapy using combined chemo-hormonal, surgical and radiation therapy in patients with primary oligometastatic hormone-sensitive PCa.
Material and methods. The study included 48 patients with primary oligometastatic prostate cancer who received combination treatment within the internal one-research-center protocol. At the first stage, all patients underwent combined drug therapy with docetaxel (75 mg/m2 intravenously every 3 weeks for 6 courses) and degarelix. Patients who had a decrease in PSA level ≤ 2 ng/ml and registered stabilization of the disease according to radiological examination were treated surgically through RPE with extended pelvic and retroperitoneal lymph node dissection. Radiation therapy was performed only in patients with the presence of bone lesions at a dose of 50-70 Gy to the location of bone metastases in the stage 3 plan of combined multimodal therapy.
Results. PCa biochemical relapse was verified in 27 (56.3%) patients during the median follow-up of 10 months. The average time to PSA increase was 9.0 ± 5.7 months (from 1 to 24 months), median — 7 months, Six-month PSA relapse-free survival (PSA-RFS) was 61.2 ± 7.5%; 1-year PSA-RFS — 38.0 ± 8.6%. The average duration before the initiation of hormonal therapy was 12 ± 6.1 months (from 3 to 27 months), median: 10 months. Six-month survival before the drug administration was 72.6 ± 6.8%; twelve-month survival: 40.9 ± 8.7%. About 40% of patients with oligometastatic PCa had no signs of progression and did not receive any other drug therapy for 12 months after completion of protocol treatment.
Conclusions. Analysis of the study results demonstrates satisfactory oncological outcomes of the studied treatment option in patients with newly diagnosed oligometastatic hormone-sensitive PCa, as well as a low likelihood of side effects and complications. Nevertheless, it is necessary to continue conducting larger and more structured randomized trials to determine the possibility of applying this therapeutic approach in clinical practice.
Keywords
For citations:
Nyushko K.M., Perepukhov V.M., Alekseev B.Ya. Multimodal therapy for oligometastatic prostate cancer: results from a single-centre study. Urology Herald. 2021;9(4):70-86. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-4-70-86
Introduction
Prostate cancer (PCa) is one of the most widespread oncological diseases worldwide. According to the International Agency for Research on Cancer, PCa occupies 4th place in the world by the rate of newly diagnosed cases of malignancies. The occurrence rate was nearly 1.4 million (7.3%) in 2020 with the lethality rate of nearly 375,000 cases [1].
The tactic of treatment for PCa is determined by the spread of the oncological process. Radical prostatectomy (RPE) and radiotherapy (RT) are the main options of radical treatment for patients with localized and regional PCa. RPE and RT are the most common methods of treatment for localized PCa. However, during the past years, researchers’ interest increased in surgical and radiotherapy not only in patients with regional and lymphogenous-disseminated but also metastatic PCa. The number of studies increased that demonstrate the improvement of the survival parameters in patients with PCa with remote metastases that underwent RPE and lymph node dissection in combination with drug therapy in comparison with a cohort of patients that received only pharmacotherapy [2]. Thus, the study of the possibility of surgical treatment and RT as a part of local multimodal treatment in combination with pharmacotherapy in patients with metastatic PCa is relevant, which will improve the survival parameters in patients.
The study aimed to evaluate the effectiveness of multimodal combined chemohormonal, surgical treatment, and RT in patients with primary oligometastatic hormone-sensitive PCa.
Materials and methods
An internal single-center study included 48 patients with newly diagnosed oligometastatic PCa that underwent combined therapy and had not received pharmacotherapy for it before. All patients in the study received the results of radiological examination. They got explained the concept of the planned therapy and proposed possible variants of treatment considering the spread of the oncological process. All patients signed a form of informed consent. The study design was approved by the Ethics Committee of the Herzen Moscow Oncology Research Institute (Protocol №12 dated May 16, 2017).
Baseline radiological investigation. All patients with oligometastatic PCa underwent examination that included bone scintigraphy, pelvis MRI scanning, transrectal ultrasonography, abdominal, and thoracic CT. Positron emission tomography (PET-CT) was not obligatory. According to the results of the examination, all patients had metastases in pelvic and/or retroperitoneal lymph nodes (LN) (≤5 foci). In 21 patients (43.7%), additional single metastatic lesions (≤ 3) were revealed in bones (spine and pelvis).
Treatment protocol design. Patients with a metastatic lesion of only LN (27 (56.3%) patients) underwent combined therapy that included neoadjuvant chemohormonal therapy (Neo-CHT). In the subgroup of patients with response to the treatment, further RPE and extended pelvic and retroperitoneal lymph node dissection (LND) were performed. Patients with single metastases in bones (21 (43.7%) patients) in combination with a metastatic lesion of LN received combined treatment that included Neo-CHT and further surgery. In 1 – 1.5 months after surgery, patients underwent External Beam Radiation Therapy (EBRT) around localization of bone metastatic foci (TFD 50-70 Gy) (Fig. 1). The proposed method of combined treatment has a patent for invention – RU 2695348 C2, 23.07.2019 [3].
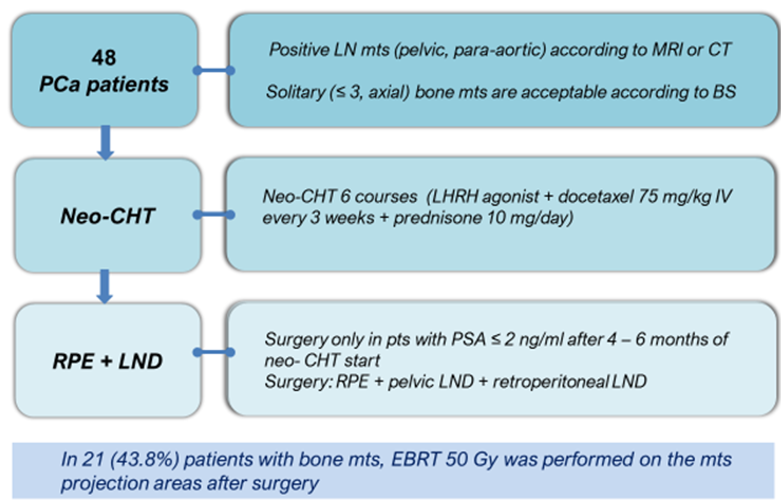
Figure 1. Study design of the protocol for combination therapy in patients with oligometastatic hormone-sensitive prostate cancer (BS — bone scan, Neo-CHT — neoadjuvant chemo-hormone therapy, LHRH — luteinizing hormone-releasing hormone, LND — lymph node dissection)
Neoadjuvant chemo-hormone therapy. Docetaxel (75 mg/m2 intravenously every 3 weeks) in combination with degarelix, luteinizing hormone-releasing hormone (LHRH) (starting dose 240 mg with further 80 mg supporting doses), for 6 courses.
Surgical treatment. All patients with response to pharmacotherapy (decrease in the level of PSA (prostate-specific antigen) ≤ 2 ng/ml and registered stabilization of the disease verified by radiological studies were prepared for the surgical stage of treatment. The surgical stage of treatment was performed 2 – 4 weeks after the end of preoperative pharmacotherapy provided the blood parameters got normalized and the condition of patients was satisfactory after pharmacotherapy. In all patients included in the analysis, surgical treatment was performed by one surgeon in 2017-2019.
The surgery started with a lower midline extraperitoneal approach from the umbilicus to the pubis, which is identical to the routine RPE. Initially, RPE was made with the standard Walsh technique. RPE had some technical peculiarities in the mobilization and removal of the prostate, which was associated with drug-induced pathomorphosis. The volume of the prostate was significantly reduced and fibrosis of periprostatic tissues was observed (Fig. 2). Still, in all the cases, prostatectomy was technically possible and was not associated with serious intraoperative complications. In all patients, RPE was followed by the extended pelvic and retroperitoneal LND to the level of the renal blood vessels (Fig. 3). The removed metastatic LN were sent separately to the routine morphological study with the specification of their localization.

Figure 2. Mobilization of the prostate during the second (surgical) stage of combined treatment in a patient with oligometastatic prostate cancer after drug treatment according to a patented technique

Figure 3. Intraoperative field view after performing extended pelvic lymph node dissection (A) and retroperitoneal (B) lymph node dissection: 1 — aorta, 2 — right common iliac artery, 3 — left common iliac artery, 4 — inferior mesenteric artery, 5 — left ureter, 6 — left iliac-lumbar muscle
External Beam Radiation Therapy. After the primary examination and post-operative rehabilitation, the patients with metastatic bone foci (≤3 metastatic foci in bones) were prepared for EBRT in the area of bone metastases. The marking and radiation were performed with any variant of 3D conformal beaming including irradiation with a modulation of the beam intensity and stereotaxic radiation for local precise exposure. Topometry was made using CT or MRI fusion. To verify the positioning, the method of portal visualization was applied. The regimen of dose fractionation was wide-field irradiation combined with a boost of 19.5/26.1 Gy (dosage per volume/total local dosage) for 3 daily fractions of 6.5/8.7 Gy each (TFD — 60 Gy).
Disease monitoring. After the end of combined therapy, all patients received dynamic follow-up to monitor PSA levels every month. In the case of biochemical recurrence or clinical progression of the disease (pain syndrome), a complex radiological study was performed. The patients did not receive any additional pharmacotherapy during the examination before the development of signs of biochemical recurrence or radiological progression of the disease. In the case of biochemical recurrence or radiological progression of the disease, patients got indicated hormonal therapy (HT). In the cases of signs of castration refractory (CR) disease (PSA increase > 2 ng/ml in more than two consecutive tests in patients with castration level of testosterone (<50 ng/dl)), the doctors defined the time to the development of castration-resistant PCa (CRCPa). Further, they underwent therapy according to the approved international recommendations.
Statistical analysis. For the description of the studied parameters, the authors used minimal and maximal values, mean values, the standard deviation of the mean. If the distribution was different from normal, median and interquartile range (25% percentile and 75% percentile), and 95% confidential interval (CI) were used. To compare the interdependent groups, non-parametric methods were used. Quantitative features were compared using the Mann-Whitney U-test (for two groups) and ANOVA Kruskall-Wallis test. Qualitative features were compared using Pearson’s χ² test and 2x2 tables. Associations between two features were assessed using Spearman correlation analysis or contingency tables (Pearson’s χ² test, Fisher’s exact test). The survival rate of patients was evaluated by the Kaplan-Meier method. Cox regression analysis was used to evaluate the influence of some features of the survival. The difference was statistically significant at p < 0.05. The data was analyzed in the software Statistica 10.0 (StatSoft Inc., IBM Corp., USA).
Results
The mean age of patients was 62.0 ± 6.4 years old (from 43 to 73 years old), median – 62 years old (when PCa was diagnosed). The mean level of the baseline PSA in patients, from the time when the diagnosis was verified and to the time of pharmacotherapy, was 84.7 ± 132.9 ng/ml (from 3.5 to 810 ng/ml), median — 36.5 ng/ml. The mean percent of positive biopsies was 80.6 ± 20.1% (33.3 – 100%), median — 83%. The mean volume of the prostate before the therapy (when the diagnosis was verified) was 49.7 ± 14.2 см3 (27 – 102 cm3), median 46 cm3. The differentiation of tumor by Gleason scale showed that 6 score (3 + 3) was observed in 1 (2.1%) patient, 7 score (3 + 4) — in 14 (29.2%) of patients, 7 score (4 + 3) — in 4 (8.3%) patients, and 8 – 10 score — in 29 (60.4 %) patients. The isolated lesion of pelvic LN (≤ 5 metastases) was revealed in 5 (10.5%) patients, pelvic and retroperitoneal LN lesions (≤ 5 foci) were verified in 22 (45.8%) patients, and bone metastatic foci (≤ 3) combined with lesion pelvic and retroperitoneal LN (total number ≤ 5 foci) was registered in 21 (43.7%) patients by the results of the radiological studies.
Each patient received 6 courses of Neo-CHT (docetaxel in combination with degarelix). The tolerability of therapy was satisfactory. Toxicity-induced manifestations were not observed in 21 (43.9%) patients. Gastrointestinal toxicity was registered in 7 (14.6%) patients, neutropenic complications were observed locally in 23 (47.9%) patients. In three (6.2%) patients, a combination of these complications was revealed. Thus, side effects of chemotherapy were observed in 27 (56.1%) patients.
The levels of PSA were 1.07 ± 0.74 ng/ml (from 0.03 to 2 ng/ml), median — 1.03 ng/ml, six months after Neo-CHT in 48 patients. A decrease in the level of PSA ≤ 1 ng/ml was observed in 23 (47.9%) patients. A decrease in PSA level reached the range from 1 to 2 ng/ml in 25 (52.1%) patients. PSA level decreased to ≤ 0.2 ng/ml after 6 courses of neoadjuvant therapy in nine (18.8%) patients (Fig. 4). Besides, due to pharmacotherapy, a reduction of the prostate volume was observed. Thus, a reduction of the prostate volume by ≥ 50% from the baseline value was registered in 17 (35.4%) patients.

Figure 4. Decrease in PSA as a percentage of initial values after 6 courses of neoadjuvant chemo-hormone therapy
The mean time of surgery after Neo-CHT was 182.2 ± 42.3 minutes, median — 175 minutes. The average volume of the blood loss was 450 ml, median — 300 ml. The degrees of intra and post-operation complications are presented in Table 1. A serious complication that required additional surgery and transfer to the ICU was registered only in one (2.1%) patient. There were no complications that led to disabilities or lethal outcomes among the studied patients.
Table 1. Complications according to the Clavien-Dindo classification
|
Severity |
n |
% |
|
I |
8 |
16.7 |
|
I |
15 |
31.3 |
|
IIIa |
5 |
10.4 |
|
IIIb |
0 |
0 |
|
IVа |
1 |
2.1 |
|
IVb |
0 |
0 |
|
V |
0 |
0 |
|
Total |
22 |
45.9 |
A routine morphological study showed that drug-induced pathomorphosis was observed in all patients that received pre-operation Neo-CHT. Weak drug-induced pathomorphosis of 1 – 2 degrees was registered in 26 (54.3%) patients. Expressed drug-induced pathomorphosis of 3 – 4 degrees was observed in 22 (45.8%) patients. The mean number of LN removed during extended pelvic and retroperitoneal LND was 42 ± 13 LN (from 20 to 65), median — 43. The mean number of the revealed metastases was 9 ± 8 LN (from 1 to 42), median — 6. The number of patients with combined metastatic lesions of pelvic and retroperitoneal LN increased by 2 times in comparison with pre-operative data. The isolated lesion of only retroperitoneal LN was not registered. Thus, the results of the routine morphological study indicated larger LN lesions in this group of patients in comparison with the results of the pre-operation examination.
All patients that received combined therapy according to the single-center protocol received post-operative follow-up and did not have additional pharmacotherapy until the appearance of the signs of the disease progression. The mean duration of follow-up was 12.4 ± 7.7 months (from 1 to 30 months), median — 10 months. Biochemical recurrence was verified in 27 (56.3%) patients during the median period. The mean time to an increase in PSA was 9.0 ± 5.7 months (from 1 to 24 months), median — 7 months; six-month biochemical relapse-free survival (PSA-BRFS) was 61.2 ± 7.5%; one-year PSA-RFS was 38.0 ± 8.6% in the subgroup of patients with the realized biochemical recurrence. The mean time to the beginning of HT was 12 ± 6.1 months (from 3 to 27 months), median – 10 months. Six-month survival before the indication of pharmacotherapy was 72.6 ± 6.8%; one-year survival — 40.9 ± 8.7%. Around 40% of patients with oligometastatic PCa did not have signs of the disease progression and did not receive any other pharmacotherapy for 12 months after the end of the therapy within the studied protocol. CRPCa was registered only in 4 (8.3%) patients during a 10-month median of follow-up. One-year CRPCa symptom-free survival was 88.6 ± 6.3%. One patient died during this period for a reason not associated with the progression of the main disease (myocardium infarction). Thus, one-year tumor-specific survival was 100% and general survival within a 19-months follow-up was 92.9 ± 6.9%.
The most significant factors of prognosis associated with biochemical recurrence were analyzed using a one-factor Cox regression analysis and presented in Table 2, and Figs. 5-11.
Table 2. Prognostic factors of PSA relapse-free survival
|
Factor |
b |
OR |
CI 95% |
p |
|
Age (≤ 60 years vs > 60 years) |
-0.224 |
0.67 |
0.35 – 1.04 |
0.009 |
|
Gleason grade ≤ 7 (3 + 4) vs ≥ 7 (4 + 3) |
0.291 |
2.1 |
1.24 – 5.15 |
0.004 |
|
Percentage of positive biopsy cores (≤ 50% vs > 50%) |
0.231 |
1.45 |
0.96 – 3.12 |
0.03 |
|
Reduction of the prostate volume after CHT (< 50% vs ≥ 50%) |
-0.211 |
0.84 |
0.46 – 1.12 |
0.02 |
|
Drug pathomorphosis (mild / severe) |
-0.271 |
0.52 |
0.22 – 0.96 |
0.005 |
|
The number of removed LN during RPE (< 30 vs ≥ 30) |
-0.188 |
0.86 |
0.52 – 1.15 |
0.04 |
|
Presence of extracapsular extension of the LN capsule (no/yes) |
0.582 |
7.12 |
4.36 – 9.32 |
0.000 |
|
Metastasis density (≤ 15% vs > 15%) |
0.596 |
8.21 |
4.15 – 12.46 |
0.01 |
|
PSA level 1 month after surgery (< 0.1 ng/ml vs > 0.1 ng/ml) |
- 0.235 |
0.52 |
0.32 – 0.99 |
0.04 |
|
Notes: 1) b — regression coefficient; OR — odds ratio; CI — confidence interval. 2) LN — lymph node; RPE — radical prostatectomy; HCT — chemo-hormonal therapy. |
||||
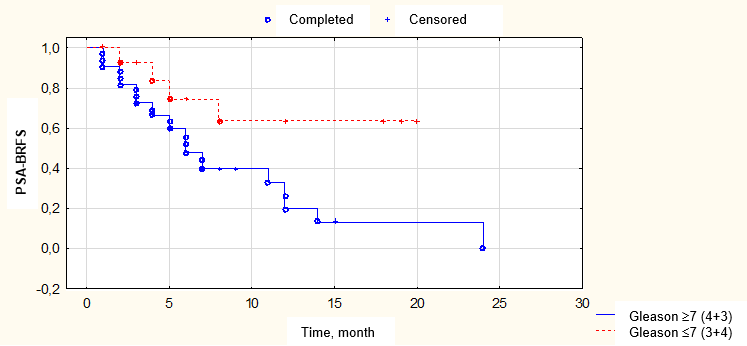
Figure 5. Biochemical relapse-free survival (BFRS) depending on tumor differentiation according to biopsy data
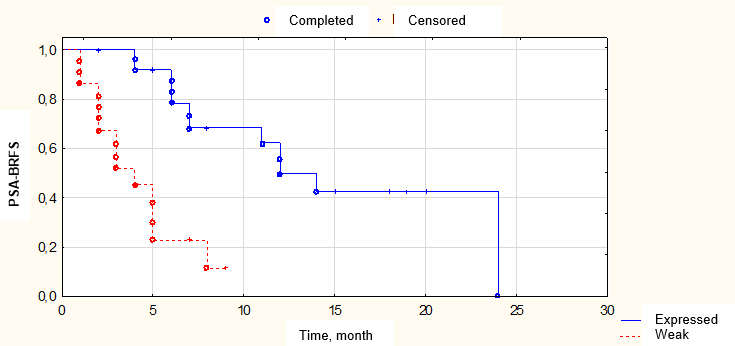
Figure 6. Biochemical relapse-free survival (BRFS) depending on the severity of drug pathomorphosis
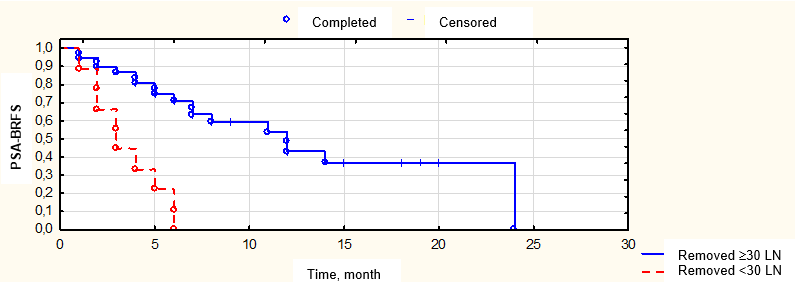
Figure 7. Biochemical relapse-free survival (BRFS) depending on the number of removed lymph nodes
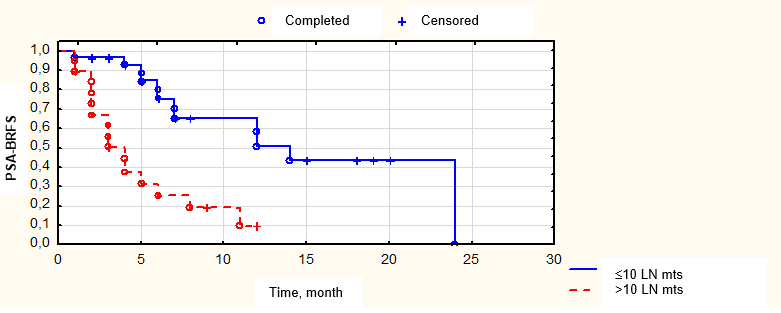
Fig. 8. Biochemical relapse-free survival depending on the number of lymph node metastases
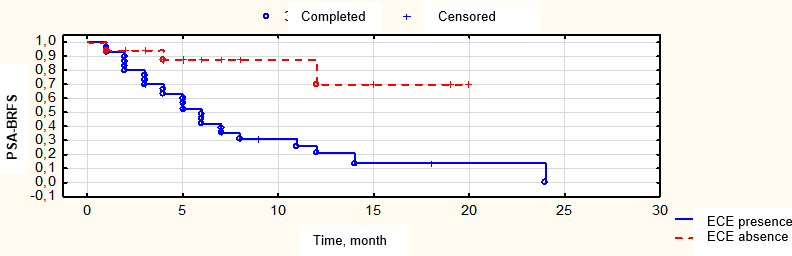
Figure 9. Biochemical relapse-free survival (BRFS) depending on the presence of tumor extracapsular extension (ECE) of the metastatic lymph node
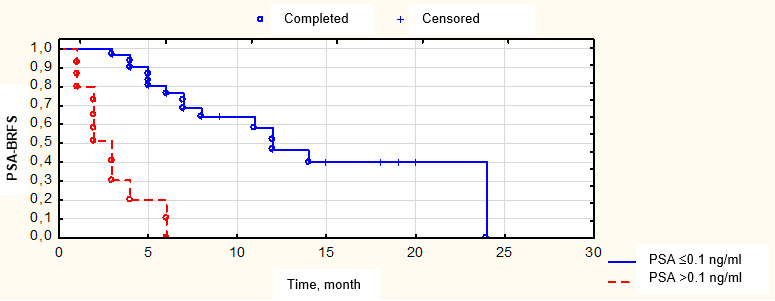
Figure 10. Biochemical relapse-free survival (BRFS) depending on the PSA level in a month after surgery
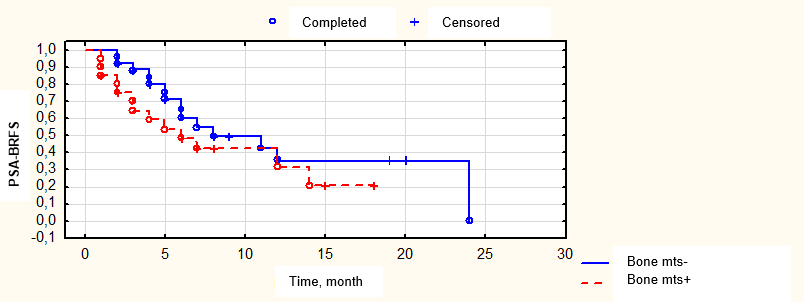
Figure 11. Biochemical relapse-free survival in patients stratified according to presence/absence of bone lesions
A decrease in the PSA levels in response to pre-operation pharmacotherapy correlated with a possibility of complete response in the PSA levels ≤ 0.1 ng/ml one month after surgery. Thus, in 23 (47.9%) patients with a registered decrease in the marker level to < 1 ng/ml after pre-operation pharmacotherapy, a complete PSA level response was observed in all 23 (47.9%) patients after the second stage of treatment. At the same time, in the subgroup of 25 (52.15%) patients with PSA levels 1 – 2 ng/ml after pharmacotherapy, a decrease in the marker level to ≤ 0.1 ng/ml was registered only in 4 (8.3%) patients after surgery (p = 0.0015). PSA-BRFS in patients with bone metastatic foci was not significantly different (p = 0.26) from the survival of patients that had only lymphogenous lesion foci (Fig. 10). The lack of differences in PSA-BRFS could be associated with EBRT applied in this cohort of patients that had a certain therapeutic abscopal effect. Still, this fact required additional studies.
According to a multi-factor Cox analysis result, only two factors had the statistically significant prognostic influence on PSA-BRFS, in particular, the degree of drug-induced pathomorphosis expression (OR = 0.13, 95% CI: 0.04 – 0.49; p = 0.002) and the level of PSA one month after the surgical treatment (ОR = 0.12, 95% CI: 0.02 – 0.51; p = 0.004).
Discussion
The results obtained in this study can indicate intriguing and promising results of the application of multimodal therapy with combined application of pharmacological, surgical, and radiation therapy in the population of carefully selected patients with hormone-sensitive metastatic PCa with a minor volume of metastatic lesions. It should be noted that metastatic PCa is an extremely heterogeneous disease. One of the most significant prognostic factors is the number of metastases and the spread of the oncological process (the volume of a metastatic lesion). Currently, there are no strict criteria for the stratification of patients with newly diagnosed metastatic hormone-sensitive PCa considering the number of metastases and their localization into cohorts of «small» and «large» volumes of metastatic lesions in patients. However, according to the results of large randomized studies that had a trivially developed stratification, the most acknowledged feature of a “large” volume metastatic lesion was the presence of visceral metastases and/or presence of ≥ 4 foci in bones during the localization of at least one bone lesions outside the area of the axial skeleton (pelvis and spine) [4]. The results of numerous randomized studies on the application of this subgroup stratification demonstrated that a cohort of patients with a large volume of the lesion and multiple metastases had the most benefit from the indicated combined pharmacotherapy with the inclusion of a standard scheme of chemohormonal therapy (docetaxel or new androgen receptor antagonists) [5][6][7][8][9][10]. Thus, it can be suggested that a subgroup of patients with oligometastatic PCa and a group of patients with «larger» volume of the lesion are quite heterogeneous considering various biological characteristics of the tumor, which is reflected in various peculiarities of the clinical course of the disease in these cohorts of patients.
Lussier et al. studied various samples of resected metastases in patients with isolated metastatic disease [11]. These authors determined various patterns of expressions of microRNA in patients with low and high metastatic activity. The authors proposed the possibility of predicting a metastatic disease using foci phenotypes for each subgroup. The results of this study confirm the hypothesis that the presence or development of oligometastatic PCa is a separate nosology unit with various biological mechanisms that differ from a disseminated process. Modern diagnostic and therapeutic possibilities provide innovative approaches to the treatment of patients with oligometastatic PCa that can change the tactics of systemic therapy and form a new strategy of combined multimodal therapy in these patients.
Some researchers already evaluated the effectiveness of new combined methods of local therapy in patients with oligometastatic PCa in the retrospective and prospective variants of the study design. Thus, two large randomized studies published in 2019 demonstrated the effectiveness of EBRT applied in radical dosage to the area of the prostate in patients with oligometastatic PCa. The obtained results showed a significant increase in the survival of patients that underwent combined multimodal treatment. This allowed the specialists to revise the existing guidelines for the treatment of patients with newly diagnosed metastatic PCa with a «small» volume of the metastatic lesion [12][13].
Besides, some studies considered the possibility of surgical treatment as a part of combined multimodal therapy in patients with PCa and clinically verified lymphogenous metastases. Zurita et al. conducted an II phase study that included 26 patients with metastases in the regional lymph nodes revealed by the results of instrumental examination before surgery (pelvic LN >2cm in diameter) or a high risk of lymphogenous progression (Gleason ≥ 8 score and PSA ≥ 25 ng/ml, cT3 and Gleason ≥ 7 score, or cT4) [14]. All patients received Neo-CHT for 12 months (docetaxel and LHRH analog ± Bicalutamide). When the levels of PSA decreased to < 1 ng/ml, patients were proposed surgical treatment that included prostatectomy and extended lymph node resection. Positive surgical margin was verified in 8% of cases and metastases in LN were revealed in 46% of patients. Complete remission was achieved (level of PSA < 0.2 ng/ml without adjuvant therapy) in 13 (50%) patients after the performed combined treatment. Time median until progression was 27 months in this group. Remission was achieved for 52.9 months in 6 patients. Nine patients died from PCa progressing during a 61-month follow-up median. The authors concluded that maximal cytoreduction after Neo-CHT and removal of pharmacotherapy-resistant tumor lesions could delay the indication of systemic therapy in patients with lympogeneous-disseminated PCa. However, these authors believe that for the development of precise recommendations on the wide implementation of a combined method of therapy into the clinical practice, large-scale randomized studies should be conducted [14]. The obtained data agrees with the data obtained in the present study during the analysis of the effectiveness of the indicated therapy in a significantly larger number of patients.
The studies on the evaluation of the effectiveness of a combined multimodal approach that includes a surgical stage in patients with oligometastatic PCa are ongoing. The main limitations of these studies are a retrospective design, a short period of follow-up, and a relatively low number of patients included in the analysis. Currently, there is only one published study (2017) with a longer period of observation on the evaluation of the surgical treatment in the subgroup of patients with newly diagnosed hormone-sensitive oligometastatic PCa, which included only 11 patients [15]. Despite a long-term follow-up period, the study results demonstrated a high rate of intraoperative complications and the necessity of adjuvant treatment in this cohort of patients. The study included patients with ≤ 5 metastatic foci in bones as well as a metastatic lesion of pelvic and/or retroperitoneal LN. The median age was 72 years old, minimal period of survival observation was 5 years. The observation period median was 63 months. Surgery time median, blood loss, and inpatient period were 170 mins, 750 ml, and 13 days, respectively. Two (18%) patients had ≥ 3 степени complications in the postoperative period. Blood tranfusion was required in eight (73%) patients. The results of the routine morphological study verified metastases in LN in ten (91%) patients. Positive surgical margin was confirmed in 88 (73%) patients. Adjuvant HT after RPE was indicated to ten (91%) patients. Still, remote results were optimistic. Seven-year progression-free survival was registered in 45%, tumor-specific lethality rate was 18%.
Heidenreich et al. (2018) conducted a larger study that included 113 patients with metastatic PCa and single metastases in bones. It demonstrated that cytoreductive RPE applied in the cohort of patients with oligometastatic PCa as one of the components of multimodal therapy is a highly effective method of local therapy for disease control and can significantly improve remote treatment results [16]. In this study, the mean duration of observation was 53.6 months (from 13 to 96 months, median 45.7 months). All patients received Neo-CHT with LHRH analogs for 3-6 months. Three-year and five-year general survival was 87.6% and 79.6%, respectively, the mean time to the development of CRPCA was 72.3 months. The study also included a correlation analysis of the influence of various prognostic factors on the parameters of survival. It was established that PSA < 1 ng/ml before surgery to the end of Neo-CHT was the most significant prognostic factor of PSA-RFS (p < 0.0004). The results of the routine morphological study showed that the application of only HT as a neoadjuvant option did not lead to expressed drug-induced pathomorphosis. Living tumor tissue with weak drug-induced pathomorphosis was revealed in all patients that received this variant of therapy. In 16 (14.2%) patients, pT4a stage was verified, in 21 (18.6%) patients — pT2a-c, and in 76 (67.3%) patients — pT3a/b. Metastases in LN were verified in 61.1% of patients, positive surgical margin — in 36.8%. In 11 (9.7%) patients, Clavien-Dindo IIIa-b complications were observed. The rate of urine incontinence was low. No urine incontinence was observed in 68.1% of patients, light degree of incontinence (1 – 2 pads per day) — in 17.7% of patients, severe degree of incontinence (>2 pads per day) — in 14.1% of patients. The authors concluded on the high effectiveness of this method in carefully selected patients that had expressed response (PSA decrease to < 1 ng/ml) to neoadjuvant therapy before surgery and the feasibility of further clinical studies in this direction.
A large-scale randomized clinical study started in September 2017. It aimed to evaluate the results of multimodal therapy in patients with oligometastatic PCa and compare these data with the application of only pharmacotherapy. The study design was finally approved by the FDA Ethical Committee in 2019. It is planned to include not less than 84 patients in the study to achieve the level of statistical significance of the analyzed results. The study will include patients with metastatic PCa stage M1a/b with 1 – 5 metastatic foci revealed by the results of the radiological study. They will be randomized into three groups. The first group will receive surgical treatment (RPE + pelvic LNE) in combination with Neo-CHT and further postoperative RT on areas of lymph efflux provided there are LN metastases verified by the results of the morphological study. The second group will contain patients with stereotaxic EBRT on the prostate and all metastatic lesions in combination with HT. And the third cohort of patients will receive only pharmacotherapy (combination of HT and abiraterone or apalutamide). The study aimed to evaluate the general and tumor-specific survival. Preliminary results of this study are expected in 2021 [17].
Mathieu et al. published a systematic review in 2019 [18]. They highlighted that despite an increasing interest to local therapy for patients with oligometastatic PCa, there are still no precise criteria for the stratification of this subgroup of patients. Different authors apply various classifications for this cohort of patients. There are no precise criteria for the number of metastases, their localization, methods of diagnostics for the evaluation of the disease spread, differentiation and aggressiveness of the tumor as well as other parameters that can significantly affect the outcome of the performed multimodal therapy. At the same time, the studies demonstrate inconsistent and controversial results. The majority of retrospective works indicate an increase in survival of patients that received surgical treatment as one of the components of multimodal therapy. According to the results of a meta-analysis, it was 55% in the group that received combined therapy with surgical treatment versus 21% in the group of patients that received only pharmacotherapy (p < 0.001). Frequently, prospective studies do not demonstrate similar results. Besides, the results of the meta-analysis showed that surgical treatment in this cohort of patients significantly reduced the risk of the development of local complications (micturition disorders, urine retention, rectal bleeding, etc) in comparison with a cohort of patients that received only systemic pharmacotherapy (10% vs 25 – 30%, respectively, p < 0.001). The analysis also included the results of two randomized studies STAMPEDE and HORRAD. They demonstrated a statistically significant increase in the overall survival in patients with oligometastatic PCa that received RT in addition to standard pharmacotherapy in comparison with the control group (ОR = 0.68; 95% CI: 0.52 – 0.90; p = 0.007). The results of other reviews also confirmed the fact that cytoreductive RPE is a relatively safe method of local therapy in patients with oligometastatic PCa with a minimum of side effects when indicated as a part of multimodal therapy in specialized oncological centers. Besides, this method of therapy is highly effective to decrease the risk of local symptoms of the disease progression. It increases the time to the development of symptoms and remote metastases. The results of some studies showed that it statistically increased the general survival of patients [12][13][19].
Currently, oligometastatic PCa remains an understudied disease. The implementation of new methods of visualization, such as whole-body MRI and PET-CT with PSMA or choline, led to a significant increase in the population of patients with newly diagnosed oligometastatic PCa. Available research data indicate the advantages of cytoreductive prostatectomy for a decrease in the risk of local complications. However, its influence on survival is unknown. RT can be more effective in comparison with only pharmacotherapy in patients with few metastases. Still, there were no randomized studies that evaluated the effectiveness of surgical and radiotherapy in this cohort of patients [12, 13, 20]. All these facts demonstrate the relevance of the systematization of the accumulated data and their consolidation. The data obtained in the conducted retrospective study indicated the high effectiveness of multimodal therapy that included local methods of treatment (surgical and radiotherapy) in combination with standard pharmacotherapy in the selected population of patients with metastatic hormone-sensitive PCa and low volume of a metastatic lesion. However, for a wide implementation of this method into clinical practice, it is necessary to conduct large-scale randomized studies.
Conclusion
The conducted study demonstrated satisfactory oncological results of the studied variant of therapy with newly diagnosed oligometastatic hormone-sensitive PCa as well as low levels of side effects and complications. However, further large-scale, and structured randomized studies are needed for the evaluation of this therapeutic approach in clinical practice.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. DOI: 10.3322/caac.21660.
2. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65(6):1058-66. DOI: 10.1016/j.euru-ro.2013.11.012.
3. Patent RF na izobretenie № 2695348 C2/23.07.2019. Bjul. № 21. Ustinova T. V., Bolotina L. V., Njushko K. M., Pajchadze A.A., Krasheninnikov A.A., Hmelevskij E. V., Kaprin A. D., Alekseev B.Ja. Sposob lechenija bol'nyh s nalichiem metastazov v limfaticheskih uzlah i oligo-metastazov v kostjah skeleta pri rake predstatel'noj zhelezy. (In Russ.). Available at: https://elibrary.ru/download/elibrary_39272235_84874246.PDF Accessed November 13, 2021.
4. Buelens S, Poelaert F, Dhondt B, Fonteyne V, De Visschere P, Ost P, Verbeke S, Villeirs G, De Man K, Rottey S, Decaestecker K, Lumen N. Metastatic burden in newly diagnosed hormone-naive metastatic prostate cancer: Comparing definitions of CHAARTED and LATITUDE trial. Urol Oncol. 2018;36(4):158.e13-158.e20. DOI: 10.1016/j.urolonc.2017.12.009.
5. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, Shevrin DH, Dreicer R, Hussain M, Eisenberger M, Kohli M, Plimack ER, Vogelzang NJ, Picus J, Cooney MM, Garcia JA, DiPaola RS, Sweeney CJ. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080-1087. DOI: 10.1200/JCO.2017.75.3657.
6. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Villers A, Azad A, Alcaraz A, Alekseev BY, Iguchi T, Shore ND, Rosbrook B, Sugg J, Baron B, Chen LF, Stenzl A. Phase 3 study of androgen deprivation therapy (ADT) with enzalutamide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC): The ARCHES trial. Journal of Clinical Oncology. 2019;37(7_suppl):687-687. DOI: 10.1200/JCO.2019.37.7_suppl.687.
7. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131. DOI: 10.1056/NEJMoa1903835.
8. Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B, Rolland F, Theodore C, Deplanque G, Ferrero JM, Pouessel D, Mourey L, Beuzeboc P, Zanetta S, Habibian M, Berdah JF, Dauba J, Baciuchka M, Platini C, Linassier C, Labourey JL, Machiels JP, El Kouri C, Ravaud A, Suc E, Eymard JC, Hasbini A, Bousquet G, Soulie M. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-58. DOI: 10.1016/S1470-2045(12)70560-0.
9. Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, Attard G, Chowdhury S, Cross W, Gillessen S, Malik ZI, Jones R, Parker CC, Ritchie AWS, Russell JM, Millman R, Matheson D, Amos C, Gilson C, Birtle A, Brock S, Capaldi L, Chakraborti P, Choudhury A, Evans L, Ford D, Gale J, Gibbs S, Gilbert DC, Hughes R, McLaren D, Lester JF, Nikapota A, O'Sullivan J, Parikh O, Peedell C, Protheroe A, Rudman SM, Shaffer R, Sheehan D, Simms M, Srihari N, Strebel R, Sundar S, Tolan S, Tsang D, Varughese M, Wagstaff J, Parmar MKB, James ND; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248. DOI: 10.1093/annonc/mdy072.
10. Wallis CJD, Klaassen Z, Bhindi B, Goldberg H, Chandrasekar T, Farrell AM, Boorjian SA, Kulkarni GS, Karnes RJ, Satkunasivam R. Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol. 2018;73(6):834-844. DOI: 10.1016/j.eururo.2017.10.002.
11. Lussier YA, Khodarev NN, Regan K, Corbin K, Li H, Ganai S, Khan SA, Gnerlich JL, Darga TE, Fan H, Karpenko O, Paty PB, Posner MC, Chmura SJ, Hellman S, Ferguson MK, Weichselbaum RR. Oligo- and polymetastatic progression in lung metastasis (es) patients is associated with specific microRNAs. PLoS One. 2012;7(12): e50141. DOI: 10.1371/journal.pone.0050141. Erratum in: PLoS One. 2013;8(6). DOI: 10.1371/annotation/2489ae5e-3650-4897-8df6-3e974ca585c4.
12. Boeve LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, Delaere KPJ, Moorselaar RJAV, Verha-gen PCMS, van Andel G. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418. DOI: 10.1016/j.eururo.2018.09.008.
13. Choudhury A, Chen RC, Henry A, Mistry H, Mitin T, Pinkawa M, Spratt DE. STAMPEDE: Is Radiation Therapy to the Primary a New Standard of Care in Men with Metastatic Prostate Cancer? Int J Radiat Oncol Biol Phys. 2019;104(1):33-35. DOI: 10.1016/j.ijrobp.2018.12.040.
14. Zurita AJ, Pisters LL, Wang X, Troncoso P, Dieringer P, Ward JF, Davis JW, Pettaway CA, Logothetis CJ, Pagliaro LC. Integrating chemohormonal therapy and surgery in known or suspected lymph node metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2015;18(3):276-80. DOI: 10.1038/pcan.2015.23.
15. Gandaglia G, Fossati N, Stabile A, Bandini M, Rigatti P, Montorsi F, Briganti A. Radical Prostatectomy in Men with Oligometastatic Prostate Cancer: Results of a Single-institution Series with Long-term Follow-up. Eur Urol. 2017;72(2):289-292. DOI: 10.1016/j.eururo.2016.08.040.
16. Heidenreich A, Fossati N, Pfister D, Suardi N, Montorsi F, Shariat S, Grubmuller B, Gandaglia G, Briganti A, Karnes RJ. Cytoreductive Radical Prostatectomy in Men with Prostate Cancer and Skeletal Metastases. Eur Urol Oncol. 2018;1(1):46-53. DOI: 10.1016/j.euo.2018.03.002.
17. Parikh NR, Huiza C, Patel JS, Tsai S, Kalpage N, Thein M, Pitcher S, Lee SP, Inouye WS, Jordan ML, Sanati H, Jafari L, Bennett CJ, Gin GE, Kishan AU, Reiter RE, Lewis M, Sadeghi A, Aronson WJ, Garraway IP, Rettig MB, Nickols NG. Systemic and tumor-directed therapy for oligometastatic prostate cancer: study protocol for a phase II trial for veterans with de novo oligometastatic disease. BMC Cancer. 2019;19(1):291. DOI: 10.1186/s12885-019-5496-5.
18. Mathieu R, Korn SM, Bensalah K, Kramer G, Shariat SF. Cytoreductive radical prostatectomy in metastatic prostate cancer: Does it really make sense? World J Urol. 2017;35(4):567-577. DOI: 10.1007/s00345-016-1906-3.
19. Marenco J, Sooriakumaran P. Current concepts in oligometastatic prostate cancer: Is there a role for radical prostatectomy? Arch Esp Urol. 2019;72(2):174-181. PMID: 30855019.
20. Dzhabarov F. R., Alnikin A. B., Tolmachev V. G. Oligometastatic prostate cancer: diagnosis and preliminary results of radiation therapy. Vestnik Urologii. 2020;8(2):55-66. (In Russ.). DOI: 10.21886/2308-6424-2020-8-2-55-66.
About the Authors
K. M. NyushkoRussian Federation
Kirill M. Nyushko — M. D., Dr.Sc. (Med), Full Prof.; Leading Researcher, National Medical Research Radiological Centre; Prof., Dept. of Oncology, Medical Institute of Continuing Education, Moscow State University of Food Production.
125284, Moscow, 3 2nd Botkinsky Dr.; 125080, Moscow, 11 Volokolamskoe Hwy.
Competing Interests:
The authors declare that there is no conflict of interest.
V. M. Perepukhov
Russian Federation
Vladimir M. Perepukhov — Resident, Dept. of Oncological Urology, Herzen Moscow Research Oncological Institute — branch of the National Medical Research Radiological Centre.
125284, Moscow, 3 2nd Botkinsky Dr.
Competing Interests:
The authors declare that there is no conflict of interest.
B. Ya. Alekseev
Russian Federation
Boris Ya. Alekseev — M. D., Dr.Sc. (Med), Full Prof.; Deputy General Director for Science, National Medical Research Radiological Centre; Head, Dept. of Oncology, Medical Institute of Continuing Education — Moscow State University of Food Production.
125284, Moscow, 3 2nd Botkinsky Dr.; 125080, Moscow, 11 Volokolamskoe Hwy.
Competing Interests:
The authors declare that there is no conflict of interest.
Review
For citations:
Nyushko K.M., Perepukhov V.M., Alekseev B.Ya. Multimodal therapy for oligometastatic prostate cancer: results from a single-centre study. Urology Herald. 2021;9(4):70-86. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-4-70-86













































