Scroll to:
Types of the prostate blood supply during super-selective embolization of prostatic arteries
https://doi.org/10.21886/2308-6424-2021-9-3-32-43
Abstract
Introduction. Pelvic arteries have various anatomy and anastomoses with other branches of the internal iliac artery (IIA). This explains the technical complexity of identification and catheterization of prostatic arteries (PA), as well as the possibility of complications associated with non-target embolization of prostatic artery anastomoses.
Purpose of the study. To analyze the most common variants of prostate blood supply and evaluate the effectiveness of methods for identifying prostatic arteries.
Materials and methods. The study included 168 patients treated from 2013 to 2021. For catheterization of the prostatic arteries, 4 – 5 Fr microconductors and 2 – 2.8 Fr microcatheters were used. For embolization, hydrogel microspheres with a diameter of 100 – 300 µm and 300 – 500 µm were used, as well as PVA microparticles with a diameter of 100 – 500 µm. Preoperatively multi-sliced computed tomography (MSCT) angiography of the pelvic organs was performed to determine the type of prostatic angioarchitectonics.
Results. The use of preoperative MSCT angiography in combination with intraoperative digital subtraction angiography made it possible to determine the variations of prostatic artery divergence and identify their anastomoses in 100% of patients (336 pelvic sides). One prostatic artery was detected in 91.4% (307) of the pelvic sides. two independent pAs in 8.6% (29) of cases. Symmetrical anatomy of the prostatic arteries on both sides was revealed in 14.3% (24) patients, the remaining 85.7% (144) patients showed asymmetry on both sides. The absence of prostatic arteries anastomoses was detected in 75.5% (254) of the pelvic sides, and in 24.4% (82) of the pelvic sides, anastomoses were detected. Interlobar intraprostatic anastomoses were found in 10.1% (34) of the pelvic sides, communication with a. dorsalis penis was detected in 8% (27) of cases, with rectal arteries in 5.3% (18) of the pelvic sides and with urinary bladder arteries in 3.6% (12) of cases. Based on the analysis of the small pelvis angioarchitectonics in 168 patients, an anatomical classification of the prostatic arteries anatomy was proposed.
Conclusion. Super-selective embolization of the prostatic arteries is a contemporary minimally invasive method of prostatic hyperplasia treatment with a high safety profile. The pelvic arteries have extremely various anatomy, as well as anastomoses with other branches of the internal iliac artery, which complicates the implementation of super-selective embolization of prostatic arteries. The combination of preoperative MSCT and intraoperative digital subtraction angiography makes it possible to identify the prostatic artery and its anastomoses in most cases.
Keywords
For citations:
Kapranov S.A., Zlatovratskiy A.G., Karpov V.K., Shaparov B.M., Kamalov A.A. Types of the prostate blood supply during super-selective embolization of prostatic arteries. Urology Herald. 2021;9(3):32-43. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-3-32-43
Introduction
Benign prostatic hyperplasia (BPH) is a widespread socially significant pathology that occurs in around 50% of men aged 60 years old and 90% of men aged 85 years old. Super-selective embolization of prostatic arteries (prostatic arterial embolization, PAE) is an effective and safe X-ray endovascular method of treatment for prostatic hyperplasia. This method is described in the Russian guidelines on the treatment for BPH and recommended for application in the USA and Europe [1][2][3][4].
Pelvic arteries can have complicated anatomy, different options of the arterial discharge as well as anastomoses with other branches of the internal iliac artery (IIA). This explains technical complications in the identification and catheterization of prostatic arteries (PAs) and the possibility of the development of complications associated with non-targeted embolization of prostatic arterial anastomoses and excessive radial load.
The authors aimed to analyze the most common options of prostatic blood supply and to evaluate the efficiency of the methods of prostatic arteries identification based on personal experience of the application of this treatment method in 168 patients.
Materials and methods
A total of 168 patients underwent PAE for prostate hyperplasia from 2013 to 2020. All surgeries were performed by one surgical team. Microguide wires 4–5 Fr and microcatheters 2–2.8 Fr were used for the catheterization of prostatic arteries. Hydrogel microspheres (diameters 100 – 300 µm and 300 – 500 µm) and PVA microparticles (diameter 100 – 500 µm) were used for arterial embolization.
The algorithm of preoperative examination included transrectal prostate ultrasound (TRUS), bladder US, residual urine, prostate-specific antigen (PSA), uroflowmetry, and IPSS-QoL questionnaire.
During the pre-operative stage, multi-sliced computed tomography (MSCT)-angiography of the pelvic organs was performed for the determination of the type of prostate angioarchitectonics. Cone-beam CT (СBCT) was not performed. At the stage of the implementation of the method, PROVISO algorithm and the installation of a target urethral catheter. When the circumstances required, intraarterial injection of microdoses of vasodilators and separation of anastomoses with a microspiral were made.
Contraindications to PAE included drug intolerance to a contrast agent, expressed atherosclerosis, and vascular abnormalities of the development in the area of bifurcation of the aorta, external, and internal iliac arteries.
Statistical analysis. The obtained results were introduced in the electronic database in MS Excel. Descriptive statistical methods included the frequency for qualitative parameters, mean, standard deviation, minimal, and maximal values for qualitative parameters. The statistical processing of the data was made in the software IBM SPSS Statistics 25 (SPSS Inc., Chicago, IL, USA)
Results
Preoperative MSCT angiography in combination with intraoperative digital subtraction angiography allowed the authors to determine the variants of prostatic artery origin in all patients (168 patients, 336 sides of male pelvis). One prostatic artery was revealed in 91.4% (307) of pelvic sides and two independent PAs were revealed in 8.6% (29) of cases. The symmetric variant of prostatic artery origin from both sides was revealed in 14.3% (24) of patients. In 85.7% (144) of patients, bilateral asymmetry was revealed.
The most common variants of PAs origins were the internal pudendal artery (IPA) (30.9%), the anterior section of the internal iliac artery (IIA) (27.9%), inferior gluteal artery (IGA) (17.2%), and obturator artery (OA) (14.3%). Less frequent anatomic options included middle rectal artery (MRA) (5.4%) and superior vesical artery (SVA) (2.3%), etc. (Table 1).
Table 1. Variants of the prostatic artery discharge according to S.A. Kapranov [5]
|
Blood supply type |
Frequency, n (%) |
|
Type I – a. prostatica from the anterior portion of a. iliaca interna |
47 (27.9) |
|
Type II – a. prostatica from a. obturatoria |
24 (14.3) |
|
Type III – a. prostatica from a. glutea inferior |
29 (17.2) |
|
Type IV – a. prostatica from a. pudenda interna |
52 (30.9) |
|
Type V – a. prostatica from a. rectalis media |
9 (5.4) |
|
Type VI – a. prostatica from a. vesicalis superior |
4 (2.3) |
|
Type VII – Other |
3 (1.8) |
Preoperative MSCT-angiography in combination with intraoperative digital angiography allowed the authors to identify anastomoses of the prostatic artery with other pelvic arteries in all patients (168 patients, 336 sides of male pelvis). There were no anastomoses in prostatic arteries revealed in 75.5% (254) of pelvic sides. In 24.4% (82) of pelvic sides, anastomoses were revealed. Interlobar intraprostatic anastomoses were detected in 10.1% (34) of pelvic sides. The communication with a. dorsalis penis was revealed in 8% (27) of cases, with rectal arteries – in 5.3% (18) of pelvic sides, and vesical arteries – in 3.6% (12) of cases.
Bilateral embolization of prostatic arteries was successfully performed in 146 (86.9%) cases. In 22 (13.1%) patients, unilateral PAE was performed due to anatomic peculiarities. Selective embolization of prostatic arteries was performed in 17 (10.1%) cases, classic super selective PAE was performed in 67 (39.9%) cases, and PErFecTED-embolization – in 84 (5%) patients.
In the early postoperative period, the most frequent complication after PAE was acute urinary retention (AUR) (28 (16.6%) patients). In 11 (6.5%) of them, trocar cystostomy was performed, and in 17 (1.2%) patients, AUR was resolved by conservative therapy. In 23 (14.2%) cases, complications were associated with unintentional embolization of anastomoses in the prostatic arteries: pain in the rectum and/or blood streaks in the feces (19 (11.3%) patients) and appearance of trophic ulcers on the penile head (5 (2.8%) patients).
Discussion
Angioarchitectonics of the pelvic organs. To improve the efficiency and decrease the rate of complications after super–selective embolization of prostatic arteries, a detailed understanding of pelvic organ angioarchitectonics is essential. Pelvic organs are fed by the branches of IIA. Usually, this is a short artery (around 3–4 cm long) that bifurcates into two major trunks (anterior and posterior). Further branching of these arteries is variable. As a rule, the posterior trunk gives origin to the superior gluteal artery (a. glutea superior), iliolumbar artery (a. iliolumbalis), and lateral sacral arteries (a. sacralis lateralis). The anterior trunk gives origin to the superior vesicular artery (a. vesicalis superior) and inferior vesicular artery (a. vesicalis inferior), obturator artery (a. obturatorius), medial rectal artery (a. rectalis media), inferior gluteal artery (a. glutea inferior), and internal pudendal artery (a. pudenda interna). Prostatic arteries (PAs, a. prostatica) have numerous variants of origin and differ by their amount and structural peculiarities [6].
Bilhim et al. studied the anatomy of prostatic arteries in the group of 75 patients and revealed that the most frequent variant of a. prostatica origin was a mutual trunk with a. vesicalis inferior (vesicoprostatic trunk, tr. vesicoprostatici) rising from the mid-third of a. pudenda interna (34.1%) [7]. Less frequent variants ranked as follows: mutual trunk with a. vesicalis superior (20.1%), originating from the mutual gluteal-pudendal trunk (17.8%), from a. obturatorius (12.6%), and originating as a mutual trunk with a. rectalis media (8,4%). Rare variants included the inferior gluteal artery (3.7%), accessory pudendal artery (1.9%), and superior gluteal artery (1.4%).
Based on the analysis of the pelvic organs angioarchitectonics in 173 patients, De Assis et al. coordinated by Carnevale proposed anatomical classification of the prostatic arteries origin (Table 2) [8].
Table 2. Anatomical classification of the prostatic artery discharge according to F.C. Carnevale [8]
|
Blood supply types |
Frequency, % |
|
Type I — Vesico-prostatic trunk emanating from the anterior portion of the AII in the common trunk with the a. vesicalis superior |
28.7 |
|
Type II — Vesico-prostatic trunk emanating from the anterior portion of the AII below the a. vesicalis superior together with the gluteo-pudendal trunk |
14.7 |
|
Type III — Vesico-prostatic trunk emanating from a. obturatorius |
18.9 |
|
Type IV — Vesico-prostatic trunk emanating from a. pudenda interna |
31.1 |
|
Type V — Other variants of arterial discharge |
5.6 |
|
Notes: AII — a. iliaca interna . |
|
Maclean et al. performed the analysis of CT angiography images of the pelvic organs of 110 patients and reported that only 48.2% of them (53/110) had a similar bilateral configuration of the prostate arteries [9]. The analysis of pelvic organ angioarchitectonics showed the applicability of the classification by Carnevale in clinical practice (Table 3; Figure 1).
Table 3. Occurrence frequency of the prostatic artery anatomical types according to F.C. Carnevale [9]
|
Assis type |
Frequency, % |
|
Type I |
17.7 |
|
Type II |
22.7 |
|
Type III |
19.1 |
|
Type IV |
36.4 |
|
Type V |
4.1 |
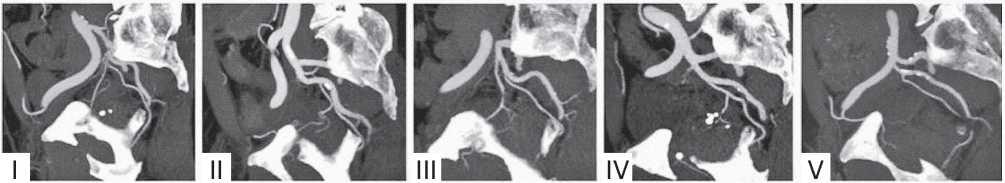
Figure 1. Anatomical variants of the prostatic artery origin according to F.C. Carnevale, types I – V. Type V in this case — the a. prostatica arises from the a. obturatoria, which emanates from the a. iliaca externa [9]
Kapranov et al. proposed 7 types of prostatic arteries. Type I: PAs originate from the anterior part of the internal iliac artery; Type II: PAs originate from the obturator artery; Type III: PAs originate from the gluteal artery; Type IV: PAs originate from the internal pudendal artery; Type V: PAs originate from the middle rectal artery [5]. Other variants of PAs origination were inited into Type VI–VII (Fig. 2). The accuracy of this classification was verified by practical observations [10, 11, 12].

Figure 2. Variants of the prostatic arteries anatomy: 1) a. prostatica from a. pudenda interna, 2) a. prostatica from a. vesicalis inferior, 3) a. prostatica from a. glutea inferior, 4) a. prostatica from a. rectalis media, 5) a. prostatica from a. obturatoria, 6) a. prostatica from a. glutea superior, 7) a. prostatica from a. vesicalis superior [5]
The prostate is usually fed by two main branches of the artery, in particular, the anterior medial or cranial branch that feeds the central part of the prostate including its middle lobe, and the posterior lateral or caudal branch that feeds the peripheral area and the top (Fig. 3). In the majority of cases, these two branches originate from a mutual trunk. However, in the majority of cases, they can arise from independent sources, which can have important technical consequences. Bilhim et al. [7] revealed one prostatic artery in 57% of cases and two independent prostatic arteries in the rest 43%.
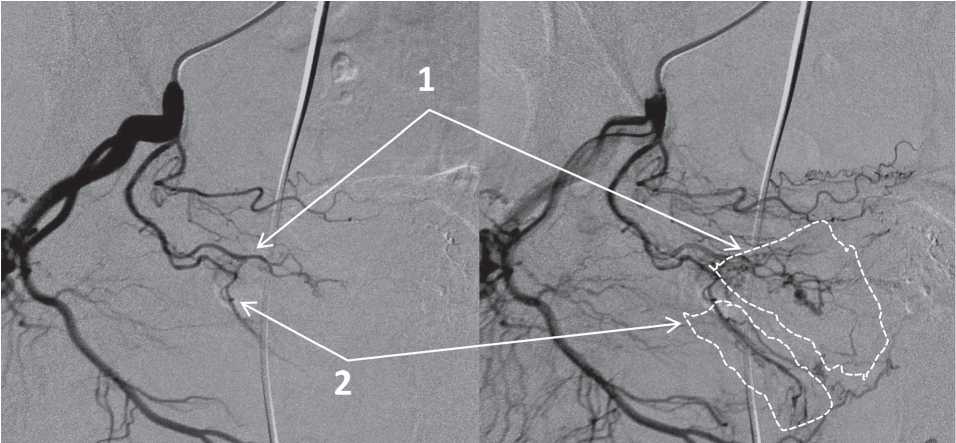
Figure 3. The cranial prostatic trunk (1) and the caudal prostatic trunk (2), areas of their blood supply
Prostatic arteries can have expressed tortuosity and be affected by atherosclerotic plaque (Fig. 4). Enderlein et al. analyzed preoperative CT angiographic images and PAE results of 104 patients with prostate hyperplasia and revealed an increase in the volume of contrast agent and time of X-ray examination in the group of patients with severe tortuosity of pelvic arteries [13]. Besides, the authors noted worsening in the technical results in the group of patients with arterial sclerotic disease of the prostatic artery mouth.
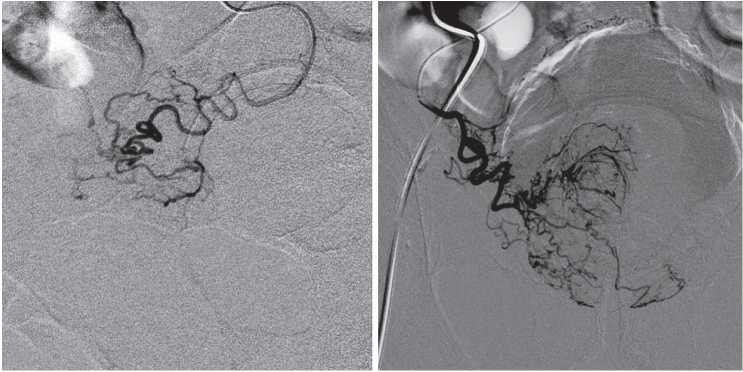
Figure 4. The severe tortuosity of the prostatic arteries
Prostatic arteries anastomoses. Prostatic arteries have anastomoses with other pelvic arteries in 57% of cases [6]. The majority of these anastomoses are characterized by a low flow rate and are visualized in the angiogram only after CT with a power–injected contrast agent. However, some anastomoses can be clinically significant because of hemodynamic patency and communication with vesical, rectal, penile, and terminal branches of the internal pudendal and obturator artery. Besides, there can be intraprostatic interlobar arterial anastomoses [14] (Figs. 5–8).
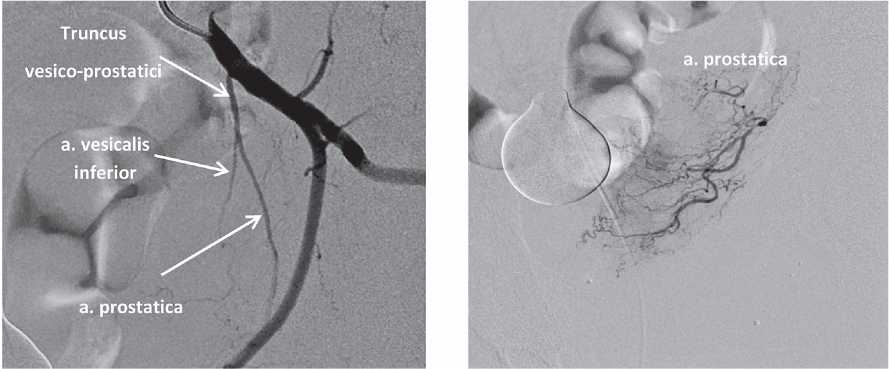
Figure 5. Anastomosis of the prostatic artery with the inferior vesical artery. The super-selective catheterization of the prostatic artery

Figure 6. Anastomosis of the prostatic artery with the rectal arteries

Figure 7. Anastomosis of the prostatic artery with the dorsal penile artery

Figure 8. The intraprostatic interlobar anastomosis (А) and anastomosis of the prostatic artery with branches internal pudendal artery (В)
Non-targeted migration of an embolizing agent through these anastomoses and retrograde discharge of embolized particles provide clinical manifestations in this group of complications.
Proper preoperative preparation, planning of surgery stages, and rational intraoperative tactics provide better results of super-selective embolization of prostatic arteries and prevents possible complications.
Methods of visualization and navigation. An intricate pelvic angioarchitectonics, variability of prostatic arteries origin, and presence of anastomoses with other arteries significantly complicate super-selective embolization of prostatic arteries. Thus, it is important to apply methods of preoperative and intraoperative visualization to increase the efficiency and safety of PAE.
To facilitate intraoperative X-ray navigation, an algorithm of identification of prostatic arteries PROVISO is used (a. pudenda interna – internal pudendal artery, a. rectalis media – middle rectal artery, a. obturatoria – obturator artery, a. vesicalis inferior – lower vesical artery, a. vesicalis superior – superior vesical artery, and obligue view). At the stage of PAE development, the installation of an indicative urethral catheter with a balloon inflated diluted X-ray contrast agent can also be used. [9][15] (Fig. 9).

Figure 9. Identification of the prostatic arteries using the PROVISO algorithm
Preoperative visualization with MSCT and MRI angiography of the pelvic organs reduces the duration of surgery and the time of fluoroscopy. Maclean et al. described the experience of comparison of MSCT-angiography with intraoperative digital subtraction angiography in the group of 110 patients [8]. Prostatic arteries were successfully identified in 97.3% of cases. The sensitivity of MSCT-angiography in the identification of anastomoses was observed in 59.0% cases, the specificity – in 94.2% of cases. Kim et al. used MRI angiography to identify PAs in 79.4% of cases, in 87% of them, the variant of origin of PAs was determined correctly [16].
Intraoperative application of flat-detector computed tomography (FD-CT) and CBCT increases the radiative load on a patient but provides better visualization of PAs anastomoses due to a combination of CT and target contrasting (Fig. 10). Wang et al. conducted a study that included 148 patients [17]. The authors revealed the PAs origin in 94.7% and its anastomoses in 97.0% of patients using CBCT. CBCT provided clinically significant data that was not available after digital subtraction angiography in 9 patients out of 148 (60.8%). Vintskovskiy et al. reported successful PAE in the group of 47 patients, in 19 of them, FD-CT was used intraoperatively [18]. After an increase in the effective dose in the group of patients with FD-CT, an improvement of the intraoperative X-ray navigation and functional results and reduction of fluoroscopy time were observed.
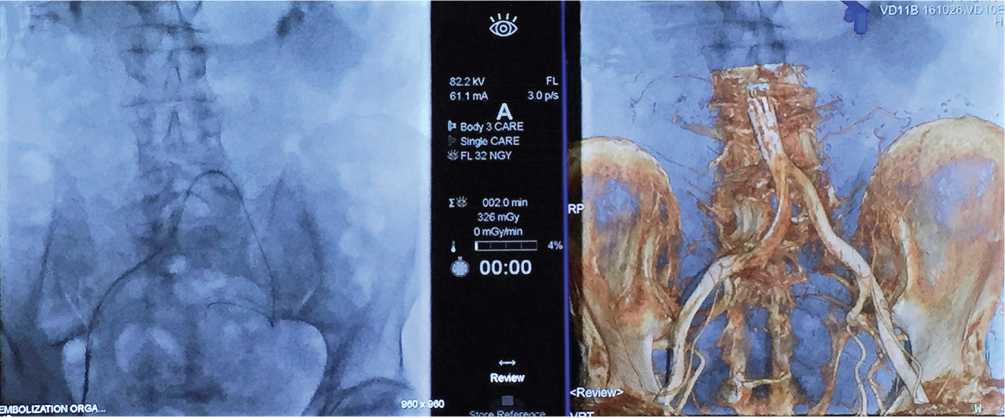
Figure 10. The parallel intraoperative application of fluoroscopy and cone-beam computed tomography with 3D-modeling for visualization of pelvic angioarchitectonics
Conclusion
Super-selective embolization of prostatic arteries is a low invasive method of therapy for prostate hyperplasia with a high safety profile. Pelvic arteries have intricate anatomy, different variants of origin, and anastomoses with other branches of IIA, which complicates super-selective embolization of PAs. A combination of a preoperative MSCT and intraoperative digital subtraction angiography provides the identification of PAs and its anastomoses in many cases.
References
1. Rasner P.I., Sivkov A.V., Kharchilava R.R. Benign prostatic hyperplasia: clinical guidelines (approved by the Ministry of Health of Russia). Moskva: Russian Society of Urology; 2020. Available at: https://cr.minzdrav.gov.ru/recomend/6_1 Accessed July 13, 2021.
2. EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam 2020. ISBN 978-94-92671-07-3
3. NICE Guidance – Prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia: © NICE (2018) Prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia. BJU Int. 2018;122(1):11-12. DOI: 10.1111/bju.14404
4. Neymark A.I., Tachalov M.A., Neumark B.A., Torbik D.V., Arzamastsev D.D. INTERVENTIONAL SURGERY IN PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA AND PROSTATE CANCER. Vestnik Urologii. 2015;(3):54-67. (In Russ.) DOI: 10.21886/2308-6424-2015-0-3-54-67
5. Kapranov S.A., Kamalov A.A., Karpov V.K., Bobrov B.Yu., Zlatovratsky A.G., Khachaturov A.A. Endovascular interventions for benign prostatic hyperplasia. In: Alekyan B.G., ed. National leadership: in 3v. X-ray endovascular surgery under the editorship. Moscow: Littera Publishing House, 2017. (In Russ.).
6. Carnevale FC, Soares GR, de Assis AM, Moreira AM, Harward SH, Cerri GG. Anatomical Variants in Prostate Artery Embolization: A Pictorial Essay. Cardiovasc Intervent Radiol. 2017;40(9):1321-37. DOI: 10.1007/s00270-017-1687-0
7. Bilhim T, Pisco JM, Rio Tinto H, Fernandes L, Pinheiro LC, Furtado A, Casal D, Duarte M, Pereira J, Oliveira AG, O’Neil JE. Prostatic arterial supply: anatomic and imaging findings relevant for selective arterial embolization. J Vasc Interv Radiol. 2012;23:1403-15. DOI: 10.1016/j.jvir.2012.07.028
8. de Assis AM, Moreira AM, de Paula Rodrigues VC, Harward SH, Antunes AA, Srougi M, Carnevale FC. Pelvic Arterial Anatomy Relevant to Prostatic Artery Embolisation and Proposal for Angiographic Classification. Cardiovasc Intervent Radiol. 2015;38(4):855-61. DOI: 10.1007/s00270-015-1114-3
9. Maclean D, Maher B, Harris M, Dyer J, Modi S, Hacking N, Bryant T. Planning Prostate Artery Embolisation: Is it Essential to Perform a Pre-procedural CTA? Cardiovasc Intervent Radiol. 2018;41(4):628-32. DOI: 10.1007/s00270-017-1842-7
10. Kamalov A, Kapranov S, Neymark A, Kurbatov D, Neymark B, Karpov V, Shaparov B. Prostatic Artery Embolization for Benign Prostatic Hyperplasia Treatment: A Russian Multicenter Study in More Than 1,000 Treated Patients. Am J Mens Health. 2020;14(3):1557988320923910. DOI: 10.1177/1557988320923910
11. Karpov V.K., Kapranov S.A., Shaparov B.M., Osmolovskiy B.E., Kamalov D.M., Kamalov A.A. Super-selective prostatic artery embolization for bph treatment. Urologiia. 2019;(3):134-41. (In Russ.). DOI: 10.18565/urology.2019.3.134-141
12. Kamalov A.A., Kapranov S.A., Neimark A.I., Kurbatov D.G., Neimark B.A., Karpov V.K, Sorokin N.I., Okhobotov D.A., Kamalov D.M., Shaparov B.M. Superselective embolization of prostate arteries in the treatment of BPH. Moscow, 2020. (In Russ.).
13. Enderlein GF, Lehmann T, von Rundstedt FC, Aschenbach R, Grimm MO, Teichgräber U, Franiel T. Prostatic Artery Embolization-Anatomic Predictors of Technical Outcomes. J Vasc Interv Radiol. 2020;31(3):378-87. DOI: 10.1016/j.jvir.2019.09.005
14. Garcia-Monaco R, Garategui L, Kizilevsky N, Peralta O, Rodriguez P, Palacios-Jaraquemada J. Human cadaveric specimen study of the prostatic arterial anatomy: implications for arterial embolization. J Vasc Interv Radiol. 2014;25(2):315-22. DOI: 10.1016/j.jvir.2013.10.026
15. Carnevale FC, Antunes AA. Prostatic artery embolization for enlarged prostates due to benign prostatic hyperplasia. How I do it. Cardiovasc Intervent Radiol. 2013;36(6):1452-63. DOI: 10.1007/s00270-013-0680-5
16. Kim AY, Field DH, DeMulder D, Spies J, Krishnan P. Utility of MR Angiography in the Identification of Prostatic Artery Origin Prior to Prostatic Artery Embolization. J Vasc Interv Radiol. 2018;29(3):307-10.e1. DOI: 10.1016/j.jvir.2017.11.001
17. Wang MQ, Duan F, Yuan K, Zhang GD, Yan J, Wang Y. Benign Prostatic Hyperplasia: Cone-Beam CT in Conjunction with DSA for Identifying Prostatic Arterial Anatomy. Radiology. 2017;282(1):271-80. DOI: 10.1148/radiol.2016152415
18. Vintskovsky S.G., Hotchenkov M.V., Uchvatkin G.V. Safe and precise embolization. The use of flat-detector computed tomography for embolization of the arteries of the prostate. Materials of the 5th scientific-practical conference of urologists of the North-West Federal District of the Russian Federation. April 18-19, 2019, St. Petersburg. (In Russ.).
About the Authors
S. A. KapranovRussian Federation
Sergey A. Kapranov - M.D., Dr.Sc.(M), Full Prof.
117997, Moscow, 1 Ostrovityanova st.
Competing Interests:
The authors declare no conflicts of interest.
A. G. Zlatovratskiy
Russian Federation
Anton G. Zlatovratskiy – M.D., Cand.Sc.(M); Head, X-ray Surgical Methods of Diagnosis and Treatment Division
119415, Moscow, 42 Lobachevsky st.
Competing Interests:
The authors declare no conflicts of interest.
V. K. Karpov
Russian Federation
Valery K. Karpov – M.D., Cand.Sc.(M), Assist.Prof. (Docent); Assoc.Prof., Dept. of Urology and Andrology, Faculty of Fundamental Medicine
119991, Moscow, 1 Leninskie gory
Competing Interests:
The authors declare no conflicts of interest.
B. M. Shaparov
Russian Federation
Boris M. Shaparov – M.D.; Postgraduate student, Dept. of Urology and Andrology, Faculty of Fundamental Medicine
119991, Moscow, 1 Leninskie gory
tel.: +7 (925) 371-91-07
Competing Interests:
The authors declare no conflicts of interest.
A. A. Kamalov
Russian Federation
Armais A. Kamalov – Academician of the Russian Academy of Sciences, M.D., Dr.Sc.(M), Full Prof.; Head, Dept. of Urology and Andrology, Faculty of Fundamental Medicine, Lomonosov Moscow State University; Headmaster, Lomonosov Moscow State University Clinic
119991, Moscow, 1 Leninskie gory
Competing Interests:
The authors declare no conflicts of interest.
Review
For citations:
Kapranov S.A., Zlatovratskiy A.G., Karpov V.K., Shaparov B.M., Kamalov A.A. Types of the prostate blood supply during super-selective embolization of prostatic arteries. Urology Herald. 2021;9(3):32-43. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-3-32-43













































