Scroll to:
The three-dimensional reconstruction of the dilated renal pelvicalyceal system by non-enhanced computed tomography
https://doi.org/10.21886/2308-6424-2021-9-3-19-24
Abstract
Introduction. The three-dimensional reconstruction of the renal pelvicalyceal system (PCS) is possible when performing enhanced computed tomography (CT). However, the use of a contrast agent has its limitations associated with the presence of allergy and chronic kidney disease.
Purpose of the study. To describe the method of semi-autonomous three-dimensional (3D) reconstruction of the PCS based on non-enhanced CT images of patients with upper urinary tract obstruction.
Materials and methods. Five patients diagnosed with renal colic were recruited from April-May 2021. All patients underwent CT-urography after informed consent. Medical Imaging Interaction Toolkit program (MITK) expanded with explainable update were used for 3D-reconstruction of PCS via excretory and native phases. To assess the accuracy of the latter, both contrast and non-contrast models were compared regarding their surface area. Also, the PCS of one patient was used to reconstruct virtual endoscopic views based on enhanced and non-enhanced models. Five urologists estimated their similarity and potential use of non-enhanced models for the interventional planning via a Likert scale questionnaire. The resulting models were also analyzed by programmer-engineers to test their suitability for 3D-printing.
Results. The average surface area of enhanced and non-enhanced models was 3291 mm2 and 2879 mm2, respectively. Obtained models were suitable for their intraluminal reconstruction and potential 3D-printing. Analyzed properties of non-enhanced models were estimated at 4.5 out of 5.0.
Conclusion. The described semi-autonomous reconstruction of the renal PCS based on non-enhanced CT images allows for a short time to reconstruct its 3D-view in patients with the upper urinary tract obstruction.
For citations:
Guliev B.G., Komyakov B.K., Talyshinskiy A.E. The three-dimensional reconstruction of the dilated renal pelvicalyceal system by non-enhanced computed tomography. Urology Herald. 2021;9(3):19-24. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-3-19-24
Introduction
Presently, non-enhanced computed tomography (CT) is a standard method of diagnostics of nephroureterolithiasis that provides preoperative visualization of the localization, density, and size of stone [1]. However, its significant drawback is the impossibility of a 3D-visualization of the pelvicalyceal system (PCS). A detailed study of the peculiarities of PCS structure (number and orientation of calyces, length and width of their necks, and an angle ratio of different parts) is required for the choice of optimal surgical tactics. Along with that, this data can be obtained by CT with intravenous contrast that allows for 3D-reconstruction before the surgery. However, this increases radiation exposure on the patient. Besides, a contrast agent is contraindicated in patients with Chronic Kidney Disease (CKD) and hypersensitivity to the drug [2]. The above-mentioned drawbacks require the development of 3D-reconstruction methods of PCS reconstruction based on contactless CT. There are scientific publications that describe methods of 3D reconstruction of renal structures by non-enhanced CT images but they target the visualization of its parenchyma [3, 4]. Only one article was dedicated to the reconstruction of PCS [5]. Besides, the isolation of the borders of the renal cavity system was made manually, which significantly elongated the time of preparation of the data and limited the application of this method into clinical practice.
Thus, the present study aimed to describe a method of semiautomated isolation of PCS in the images of non-enhanced CT with further 3D-reconstruction.
Materials and methods
A total of 5 patients with renal colic were recruited from April to May 2021. Ultrasound investigation was performed and pyelocalycoectasia was revealed. Patients signed a form of informed consent for a contrast CT with Omnipac 300.0 on a 64-slice CT scanner with 0.5 mm slice thickness Somatom Definition AS. The obtained images were studied in the program Medical Imaging Interaction Toolkit (MITK) that was used for 3D-reconstruction of PCS by the images of excretory phase and creation of a standard virtual model. Further, three random points were designated on each slice of the non-enhanced CT phase for the evaluation of the density differences around these points and determination of PCS boundaries without the need in their manual isolation (Fig. 1).

Figure 1. Designation of points on the axial non-enhanced CT slices within the PCS: A — setting the first point; B — setting the second point; C — setting the third point and automated determination of the PCS boundaries
Further, automated mergence of the selected areas was performed. Due to a polygonality of the obtained 3D-constructions, the same algorithm was used for smoothing of the surface (Figs. 2-3).
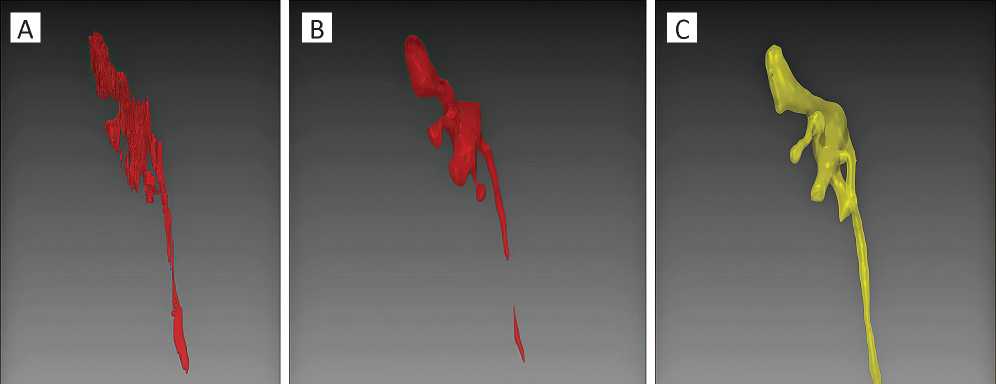
Figure 2. An example of virtual models, side view: A — automated PCS reconstruction using non-enhanced CT images before smoothing; B — non-enhanced 3D-reconstruction of the PCS after smoothing; C — 3D-reconstruction of the CT excretory phase
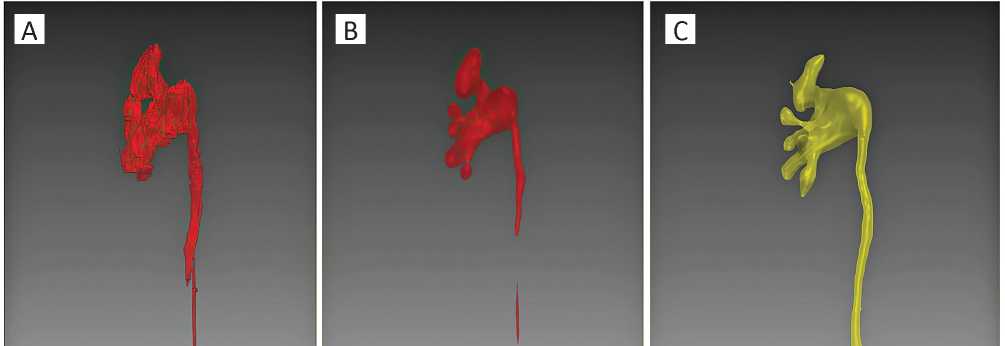
Figure 3. An example of virtual models, front view: A — automated PCS reconstruction using non-enhanced CT images before smoothing; B — non-enhanced 3D-reconstruction of the PCS after smoothing; C — 3D-reconstruction of the CT excretory phase
The volume of the obtained virtual models and the volume of contrast reconstructed models were compared. Five urologists evaluated the informative value of such reconstructions for the investigation of the PCS anatomy of a certain patient. The obtained models were also analyzed by IT specialists for suitability for 3D-printing.
Statistical analysis. The statistical analysis was made in the IBM SPSS Statistics 22.0 software (SPSS Inc., Chicago, IL, USA). The evaluation of nominal data was made with a chi-square test. The difference was significant at p < 0.05.
Results
In all cases, the duration of PCS boundaries determination and its reconstruction with smoothing was less than 10 minutes. The mean surface area of contrast and non-enhanced 3D models was 3291 mm2 and 2879 mm2, respectively (p = 0.12). The comparison of contrast and non-enhanced 3D models as well as their evaluation and feasibility for preoperative planning scored 4.5 out of 5.0 by urologists. A discussion with engineers on the adequacy of the models for 3D-printing showed that all PCS parts were sufficiently visualized and did not require correction. Potentially weak parts of the printed-out models, in particular, minor calyx necks, did not require the formation of additional supports before printing. Thus, the models were completely suitable for potential 3D-printing.
Discussion
Non-enhanced CT provided a urologist with reliable information on the main parameters of ureteral stones that include size, site, and density. However, it is impossible to study in detail the renal cavity system, which requires additional diagnostic procedures for reliable planning of possible surgery. Up to now, 3D-reconstruction was possible only after contrast CT that has an excretory phase in 3-5 minutes. The high density of the contrast is comparable with the density of bone structures. And the filling of PCS provides a 3D reconstruction via an automated overlay of CT images. As a result, a urologist gets a possibility to evaluate all structural peculiarities of the renal cavity of a certain patient, in particular, the number and orientation of the minor calyces, length, and width of the necks, as well as the angle ratio between different parts of PCS. In turn, this information allows a specialist to choose proper tactics of the surgery for nephrolithiasis increasing the efficiency of the intervention and decreasing the rate of associated complications [6].
The application of a contrast agent has such disadvantages as an increase in radiative exposure and load on a patient’s organism and a dependence on the patient’s state of health, in particular, impairments of renal excretory function. This makes the development of 3D methods of reconstruction by non-enhanced images a relevant task.
Automated reconstruction is possible for structures with a relatively constant shape without branching, like heart chambers [7], aorta [8], intracerebral hemorrhage [9], and kidney parenchyma [10]. This approach is not feasible for the renal cavity because of its variable and branchy structure, small differences in the density of the surrounding structures (vessels, adipose tissue), and collapsed shape of the renal cavity in the norm. The last aspect prevents the development of available solutions for previous limiting factors. The authors’ point of view is shared only in one publication (Sung et al.) on the description of 3D-reconstruction of PCS by the images of non-enhanced CT [5]. Sung et al. proposed a protocol of intravenous infusion with diuretic load for artificial dilation of PCS before CT scanning. This approach showed to result in a significant volume of the renal cavity system reconstruction in comparison with the reconstruction of images made in the control group. The determination of PCS boundaries was performed manually by an IT specialist, which took more than 20 minutes and required sufficient experience. This limited the availability of this approach for routine application in medical institutions.
The present work included patients with renal colic that underwent CT scanning during the episode of obstruction that lead to a dilation sufficient for the visualization of the collecting system of a kidney. The determination of the boundaries was performed in a semi-automated mode. Several random points were designated within the boundaries of PCS in each non-enhanced CT image for the determination of its boundaries. After the mergence of the images, a 3D model was built. Further, the described algorithm automatically determined PCS boundaries significantly reducing the time of determination (to 5 minutes). This method did not require specialized experience and the procedure of segmenting could be performed by a urologist.
It should be mentioned that there were limitations in the present study. Firstly, the analysis included CT scans of five patients, which did not allow for the approbation of the described algorithm in the reconstruction of all variations of PCS. Secondly, the selected patients had nephrolithiasis complicated by the obstruction of the upper urinary tracts with dilation of PCS. Thus, this approach proved feasible in this cohort of patients. And thirdly, the reconstruction was performed in a semi-automated mode, which also required the involvement of the specialist in its realization. Finally, the described algorithm was not tested in patients with the presence of a stone in the PCS, which could negatively affect the precision of the automated determination of the boundaries. Further improvement of this algorithm will target a complete automated performance that will select the borders of PCS in all CT slices after the designation of one point.
Conclusion
A semi-automated reconstruction of the PCS by non-enhanced CT scans provides its possible visualization without enhancement in patients with an obstruction of the upper urinary tract.
References
1. Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13(11):654-62. DOI: 10.1038/nrurol.2016.154
2. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, hilton S, Reese PP. The Controversy of contrast-Induced nephropathy with Intravenous contrast: What Is the risk? Am J Kidney Dis. 2020;75(1):105-13. DOI: 10.1053/j.ajkd.2019.05.022
3. Shimizu A, Ohno R, Ikegami T, Kobatake H, Nawano S, Smutek D. Segmentation of multiple organs in non-contrast 3D abdominal CT images. Int J CARS. 2007;2:135-42. DOI 10.1007/s11548-007-0135-z
4. Parkhomenko E, O’Leary M, Safiullah S, Walia S, Owyong M, Lin C, James R, Okhunov Z, Patel RM, Kaler KS, Landman J, Clayman R. Pilot assessment of Immersive virtual reality Renal Models as an Educational and Preoperative Planning Tool for Percutaneous Nephrolithotomy. J Endourol. 2019;33(4):283-8. DOI: 10.1089/end.2018.0626
5. Sung JM, Jefferson FA, Tapiero S, Patel RM, Owyong M, Xie L, Karani R, Ghamarian P, Lall C, Clayman RV, Landman J. evaluation of a diuresis enhanced non-contrast computed tomography for kidney stones protocol to maximize Collecting System Distention. J Endourol. 2020;34(3):255-61. DOI: 10.1089/end.2019.0719
6. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU guidelines on Interventional treatment for urolithiasis. Eur Urol. 2016;69(3):475-82. DOI: 10.1016/j.eururo.2015.07.041
7. Shahzad R, Bos D, Budde RP, Pellikaan K, Niessen WJ, van der Lugt A, van Walsum T. Automatic segmentation and quantification of the cardiac structures from non-contrast-enhanced cardiac CT scans. Phys Med Biol. 2017;62(9):3798-813. DOI: 10.1088/1361-6560/aa63cb
8. Sedghi Gamechi Z, Bons LR, Giordano M, Bos D, Budde RPJ, Kofoed KF, Pedersen JH, Roos-Hesselink JW, de Bruijne M. Automated 3D segmentation and diameter measurement of the thoracic aorta on non-contrast enhanced CT. Eur Radiol. 2019;29(9):4613-23. DOI: 10.1007/s00330-018-5931-z
9. Patel A, Schreuder FHBM, Klijn CJM, Prokop M, Ginneken BV, Marquering HA, Roos YBWEM, Baharoglu MI, Meijer FJA, Manniesing R. Intracerebral Haemorrhage Segmentation in Non-Contrast CT. Sci Rep. 2019;9(1):17858. DOI: 10.1038/s41598-019-54491-6
10. Khalifa F, Elnakib A, Beache GM, Gimel’farb G, El-Ghar MA, Ouseph R, Sokhadze G, Manning S, McClure P, El-Baz A. 3D kidney segmentation from CT images using a level set approach guided by a novel stochastic speed function. Med Image Comput Assist Interv. 2011;14(3):587-94. DOI: 10.1007/978-3-642-23626-6_72
About the Authors
B. G. GulievRussian Federation
Bakhman G. Guliev – M.D., Dr. Sc. (M), Full Prof.; Prof., Dept. of Urology, Mechnikov North-West State Medical University; Head, Urology Centre with Robot-assisted Surgery
191015, St. Petersburg, 41 Kirochnaya st.
191014, St. Petersburg, 56 Liteiny ave.
tel.: +7 (921) 945-34-80
Competing Interests:
The authors declare no conflicts of interest.
B. K. Komyakov
Russian Federation
Boris K. Komyakov – M.D., Dr. Sc. (M), Full Prof.; Head, Dept. of Urology
191015, St. Petersburg, 41 Kirochnaya st.
Competing Interests:
The authors declare no conflicts of interest.
A. E. Talyshinskiy
Russian Federation
Ali E. Talyshinskiy – Postgraduate student; Dept. of Urology
191015, St. Petersburg, 41 Kirochnaya st.
Competing Interests:
The authors declare no conflicts of interest.
Review
For citations:
Guliev B.G., Komyakov B.K., Talyshinskiy A.E. The three-dimensional reconstruction of the dilated renal pelvicalyceal system by non-enhanced computed tomography. Urology Herald. 2021;9(3):19-24. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-3-19-24













































