Scroll to:
Urinary fistulas after partial nephrectomy in renal cell carcinoma
https://doi.org/10.21886/2308-6424-2021-9-2-111-124
Abstract
Urinary fistulas after partial nephrectomy are rare complications of this operation. There are various reasons for their development, prevention and treatment. This review analyzes the results of the cases' prevalence of urine leakage after partial nephrectomy with various approaches, occurrence's predictors of urinary fistulas, possible ways of their intraoperative prevention and treatment methods. The obtained data show that the size of tumors, their endophytic nature and proximity to the kidney pelvicalyceal system, as well as suturing of its defect, can be predictors of the development of urinary fistulas (UFs). Some authors point to the influence of long ischemia time and high blood loss on the occurrence of UFs. The main method of treating UFs is ureteral stenting or percutaneous drainage of the kidney pelvicalyceal system. For long-term persistent UFs, the method of choice can be simultaneous introduction of 2 stents, retrograde or percutaneous injection of fibrin glue, percutaneous cryoablation of the UFs.
Keywords
For citations:
Guliev B.G. Urinary fistulas after partial nephrectomy in renal cell carcinoma. Urology Herald. 2021;9(2):111-124. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-2-111-124
Introduction
Kidney tumors can be presently diagnosed at early stages due to the wide implementation of modern visualization methods. For this reason, the majority of patients hospitalized with this pathology have the T1a stage of the disease, and rarer, the T1b stage. According to the recommendations of international urological associations, partial nephrectomy (PN) became the main method of surgical treatment for kidney tumors smaller than 4 cm [1][2]. Nephron-sparing surgery provides oncologic results identical to radical nephrectomy but decreases the risk of the development of chronic kidney disease (CKD) and cardiovascular diseases [3][4][5]. At the same time, PN remains a technically complicated surgical intervention with typical complications that do not occur in patients that undergo radical nephrectomy. They are associated with technical peculiarities of PN when it is necessary to clamp the renal artery, resect the tumor, and suture the calyx and parenchymal renal defect [6, 7, 8, 9]. At each of these stages, problems can occur that lead to a decrease in kidney functioning due to long-term warm ischemic time (WIT), intra- and post-operative hemorrhage, as well as the formation of arteriovenous or urinary fistulas. Thus, in some patients, long-term benefits after nephron-sparing surgery can be overshadowed by various complications.
Urinary fistulas (UFs) after PN are defined as constant discharge in the drainage that corresponds to the urine by the chemical analysis. They cannot be referred to as life-threatening complications after PN, but they can prolong the period of hospitalization, affect the quality of patients’ life, and lead to social and domestic problems. The rate of the development of UFs varies significantly and often depends on the surgical access for PN. According to available published data, UF occurrence rate varies from 0.5 to 17.4% [10][11][12][13][14][15]. A high rate of UFs was usually observed after open PN (OPN). The implementation of laparoscopic PN (LPN) and robot-assisted PN (RAPN) decreased the rate of UFs. However, there were certain causes of a high rate of urine leakage after OPN that included the large size of the resected tumors, lack of modern suturing materials, and Hem-o-lock clips. Laparoscopic and robot-assisted techniques also significantly improved intraoperative visualization and simplified the procedure of resection. In the published literature, there are no precise predictors of the development of UFs and no common opinion on the management tactics for such patients, especially, with long-term hard-to-heal fistulas. The issues that are described in published literature provided the rationale for the present literature review.
The study aimed to make a literature review for the specification of the prevalence and causes of UFs after PN and therapeutic management for patients with this pathology.
Literate search algorithm
The author searched for articles on the diagnostics and treatment for UFs after PN in the PubMed database. The keywords included “partial nephrectomy”, “nephron-sparing surgery”, “urinary leak”, and “urinary fistula”. In general, the author collected 136 articles. Further, 54 articles were excluded because they contained the data on less than 100 PNs, no preoperative patient data, and they were not published in English. Additionally, 6 studies were excluded because they did not provide data on the incidence rate of urinary leakage or were written in a form of comments on other articles. After the mentioned search and article selection, the author included 76 articles in the present study (Fig. 1).
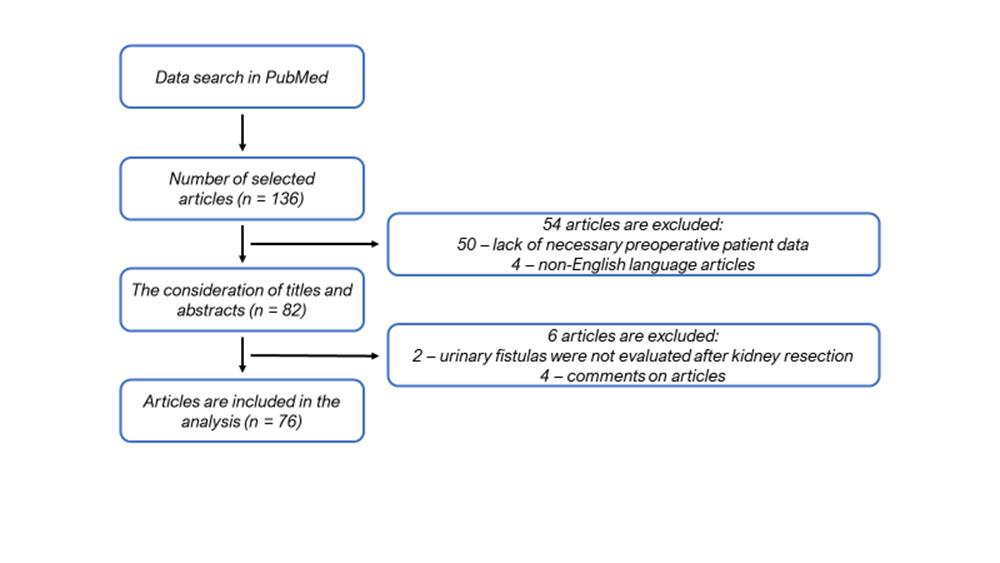
Fig. 1. Selection algorithm for a non-systematic review
The prevalence of urinary fistulas after partial nephrectomy
There are articles on different incidence rates of UFs in patients after PN. These parameters significantly differ depending on the period of publications and the used surgical access for PN. As it was mentioned above, the highest incidence rate of UFs was observed in patients after open surgery for kidney cancer [12][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30]. The data provided by different authors on the prevalence of UFs after OPN are presented in Table 1. It can be seen that after OPN, the incidence rate of UFs varies from 1.0 to 17.4%. The highest rates are provided by Сampbell et al. [18] (17.4%) and Tomaszewski et al. [30] (11.8%). It should be noted that in some early publications, despite a larger size of tumors (≥ 5.5 cm), the rate of UFs was comparatively low. Thus, Steinbach et al. [16] observed 2.1% of UFs per 140 PNs, and Moll et al. [17] – 6.7% per 164 patients. According to Meeks et al. [11], the prevalence of UFs was 13.3% (21 out of 127), including 10.5% after LPN and 18.5% after OPN.
Table 1. The prevalence of urinary fistulas after open partial nephrectomy
|
Authors, year of publication |
Number of patients |
Average tumor size (range), cm |
Incidence rate of urinary fistulas, % |
|
Steinbach et al., 1992 [16] |
140 |
5.5 (2 – 11) |
2.1 |
|
Moll et al., 1993 [17] |
164 |
5.9 (1.5 – 20) |
6.7 |
|
Campbell et al., 1994 [18] |
259 |
– |
17.4 |
|
Lerner et al., 1996 [19] |
185 |
4.1 |
1.8 |
|
Gill et al., 2003 [20] |
100 |
3.3 |
1.0 |
|
Stephenson et al. 2004 [6] |
361 |
2.5 (0.5 – 11) |
6.6 |
|
Sánchez-Ortiz et al., 2004 [21] |
184 |
3.1 (0.6 – 10) |
7.6 |
|
Thompson et al., 2005 [12] |
823 |
3.1 (0.6 – 10) |
1.5 |
|
Fergany et al., 2006 [22] |
400 |
4.18 |
9.0 |
|
Patard et al., 2007 [24] |
1048 |
3.4 |
3.1 |
|
Gill et al., 2007 [25] |
1029 |
3.3 (0.13 – 9.0) |
2.3 |
|
Kundu et al., 2007 [13] |
1023 |
2.6 |
4.6 |
|
Redshaw et al., 2014 [29] |
175 |
3.7 |
1.1 |
|
Tomaszewski et al., 2014 [30] |
355 |
3.7 |
11.8 |
In 2005, Thompson et al. [12] published series of OPN cases (1985–2001) from the Mayo Clinics. Earlier (1985–1995), the prevalence of this complication was 2.6% among 343 operated patients. However, in later series, UFs were diagnosed only in 0.6% out of 480 patients. The authors associate a large surgical volume and the improvement of preoperative visualization and surgical technique with the minimal rate of UFs occurrence. Besides, their results reflect a general tendency to a decrease in the prevalence of the urine leakage observed due to the evolution of surgical accesses (from open surgery to LPN and RAPN).
The implementation of laparoscopic and robot-assisted technologies resulted in a significant decrease in the occurrence rate of UFs. The data published by the authors on the rate of UFs after LPN [31][32][33][34][35][36][37][38][39][40][41][42] and RAPN [43][44][45][46][47][48][49][50][51][52][53] are presented in Table 2. In 2007, Gill et al. [25] reported on 1800 operated patients with the occurrence rate of UFs 2.3% after OPN and 3.1% after LPN. In 2012, Stroup et al. [26] compared the results of the treatment after OPN, LPN, and RAPN, and the rate of the urine leakage was 9, 8.3, and 3.2%, respectively. After LPN, the rates of UFs also varied greatly from 1.6 to 16.5% [31][32][33][34][35][36][37][38][39] (Table 2). In one of the first series on LPN, Venkatesh et al. [31] observed 10.7% cases of UFs in 123 operated patients, i.e., nearly in every tenth of them. At the same time, the average diameter of the tumor was small (2.6 cm) (from 1 to 9 cm). This fact could be explained by the beginning of the era of LPN and the insufficient experience of surgeons. The accumulation of the experience and increase in the rate of operated patients with the same tumor sizes led to a significant decrease in the occurrence rate of UFs. Thus, Gill et al. [25] observed this complication in 3.1% out of 771 patients, and Breda et al. [33] – only in 1.6% out of 1347 patients. However, in 2011, the later publication of Wang et al. [39] contained a high rate of UFs (16.5% among 236 operated patients). The nephrometric scales RENAL and PADUA for the evaluation of the severity of renal tumors were proposed in 2009. Thus, the authors did not have tools for the estimation of this parameter after OPN and LPN, and its influence on the rate of UFs.
Table 2. The incidence rate of urinary fistulas after laparoscopic and robot-assisted partial nephrectomy
|
Access |
Authors, year of publication |
Number of patients |
Average tumor size (range), cm |
Frequency of urinary fistulas, % |
|
LPN |
Venkatesh et al., 2006 [31] |
123 |
2.6 (1 – 9) |
10.7 |
|
Gill et al., 2007 [25] |
771 |
2.6 (0.4 – 8.0) |
3.1 |
|
|
Breda et al., 2007 [33] |
1347 |
2.8 (2 – 4) |
1.9 |
|
|
Celia et al., 2008 [34] |
592 |
2.2 (2 – 4) |
2.1 |
|
|
Lifshitz et al., 2010 [38] |
184 |
2.5 |
1.6 |
|
|
Gill et al., 2010 [37] |
800 |
3.1 |
2.4 |
|
|
Wang et al., 2011 [39] |
236 |
– |
16.5 |
|
|
Bylund et al., 2011 [42] |
104 |
2.8 (0.7 – 7.0) |
1.9 |
|
|
Stroup et al., 2012 [26] |
100 |
2.4 |
3 |
|
|
Wheat et al, 2013 [7] |
336 |
2.8 |
3.6 |
|
|
RAPN |
Rogers et al., 2008 [43] |
148 |
2.8 (0.8 – 7.5) |
1.4 |
|
Benway et al., 2010 [45] |
183 |
2.9 (1.0 – 7.9) |
1.1 |
|
|
Kaouk et al., 2011 [47] |
252 |
3.1 |
1.5 |
|
|
Dulabon et al., 2011 [48] |
446 |
(0.7 – 11.0) |
1.6 |
|
|
Ficarra et al., 2012 [49] |
347 |
2.8 |
0.6 |
|
|
Tanagho et al., 2013 [50] |
886 |
2.8 |
1.1 |
|
|
Tomaszewski et al., 2014 [30] |
476 |
3.7 |
2.5 |
|
|
Potretzke et al., 2016 [15] |
1791 |
3.0 without UFs 3.7 with UFs |
0.78 |
|
|
Connor et al., 2020 [52] |
395 |
– |
0.25 |
|
|
Peyton et al., 2020 [53] |
975 |
– |
2.5 |
|
|
Notes: LPN – laparoscopic partial nephrectomy; RAPN – robot-assisted partial nephrectomy; UFs – urinary fistulas. |
||||
The implementation of RAPN led to a minimization of the occurrence rate of UFs. In patients with medium sizes of kidney tumor (2.8–3.7 cm), this complication was observed in 0.6–3.0%. The highest occurrence rate of UFs was observed by Tomaszewski et al. [30] (in 2.5% out of 476 patients) and Zargar et al. [14] (in 3.0% out of 1019 patients). Potretzke et al. [15] published a large modern series on RAPN in 1791 patients with a 0.78% occurrence rate of UFs. This rate was significantly lower than in publications on OPN and LPN. Other series of RAPN also had low rates of urine leakage that varied from 0.6 to 2.5% [40][43][44][45][46][47][48][49][50][51][52]. In 2020, Peyton et al. [53] published that among 975 PNs performed by open and robot-assisted accesses, UFs were observed only in 2.5% of cases. Connor et al. [52] observed UFs only in 0.25% out of 395 RAPNs. Connor et al. [52] also estimated the rate of postoperative arterial malformations (AMs) and UFs after 395 RAPNs (225 patients were included in the retrospective group and 170 patients – in the prospective group). The authors analyzed demographic data, perioperative variables, and postoperative complications by the Clavien-Dindo classification. Urine leakage was defined as drainage discharge with a high level of creatinine, and AM was identified based on the postoperative visualization that indicated arteriovenous fistula or pseudoaneurysm. The predictors for these complications were evaluated with univariate analysis. There were 50% of patients with nephrometry scores of 4–6 in the first group and 31.7% – in the second group. There were nearly the same number of patients (45 and 51.2%) in both groups with the 7–9 score. There were more patients in the second group with the 10–12 score (5 and 17.1%). In other words, there were fewer patients with complicated tumors in the retrospective group. Along with the experience accumulation, there was a significant decrease in the mean operation time (141.8/112.8 min), WIT (19.7/13.4 min), and the mean volume of blood loss (182.4/129.4 ml). It should be noted that in group I, the rate of opening of the pyelocalyceal system (PCS) was significantly lower than in the prospective group (19.1%/57.1%, р < 0.002), which could be explained by a high rate of complicated tumors. The general rate of postoperative complications was 5.6%, and the cohort rate was 5.3 and 5.8%. In the retrospective group, there was a higher rate of complications classified as IIIa: 8/13 (61.5%) versus 2/8 (25%). The general rate of AM was 2.3% and the cohort rate was 3.1% (7/225) versus 1.1% (2/170). UFs were observed in 0.25% of cases; the cohort rate was 0.55% (1/225) and 0.0% (0/170). The difference in the rate of both complications between the groups was significant (р < 0.05). The results of the study showed that the implementation of RAPN significantly increased the rate of patients with UFs, which can be explained by several actors. The robot-assisted surgical system provides improved 3D visualization and a wider range of the movements of the tools, which simplified intracorporeal suturing. The application of Hem-o-lock clips for renorraphy provides significant tension during PCS and upper parenchyma defect closing.
Risk factors for urinary fistulas
Prognostic factors of the possible development of UFs after PN were studied by various authors. Thus, Meeks et al. [11] studied the causes of UFs in 127 patients after OPN and LPN. Patients were preliminarily installed an external ureteral J-stent for a retrograde introduction of methylene blue for the identification of PCS and restoration of its consistency. The general rate of UFs was 13.3% (21 out of 127). These patients had significantly larger tumors (3.19 versus 2.26 cm, р = 0.04) that were primarily endophytic (57% versus 19%, р = 0.0003). The authors also established a significant association between the restoration of the PCS defect during PN and the development of UFs (95% versus 56%, р = 0.0007). There was no association between the urine leakage and the number of the removed tumors, expected blood loss, WIT, or other surgical complications. Meeks et al. [11] reported that in 21 patients, the mean duration of urinal leakage was 53 days (median 20, range from 5 to 240). Seventeen patients had stable drainage discharge (80%), 2 had a fever, and 2 had wound infection after the removal of the drainage.
Kundu et al. [13] analyzed the results of 1118 PNs and concluded that 3 factors were significantly associated with the development of UFs: tumor size, the volume of the blood loss, and WIT. Patients with tumors > 2.5 cm were two-fold more prone to the development of UFs. The volume of the blood loss and WTI, which are often considered surgical difficulties, were also significantly associated with this complication. However, this work did not reveal a statistically significant association between PCS damage and the closing of its defect. More than 95% of patients who had urinary leakage in this series had persistent urine leakage via drainage that was installed in the pararenal space right after PN. Delayed development of UF was observed in <5% of patients, and the total rate of delayed urine leakage was observed in less than < 5%. The total rate of delayed urine leakage in all patients was 0.4%.
Univariate analysis conducted by Zargar et al. [11] showed that laparoscopic access, the presence of moderate and severe CKD, a tumor close to the PCS or renal sinus, the volume of the blood loss, and application of a ureteral catheter increased the risk of the development of UFs after the surgery. The multivariant analysis showed that tumor proximity to the PCS (р = 0.003), a beginner surgeon’s initial experience (р = 0.001), moderate and severe CKD (р = 0.04), and the volume of blood loss (р = 0.003) were primarily associated with this complication. Thus, intraoperative ureteral catheterization did not influence significantly the development of this complication (р = 0.67). The presence of moderate and severe CKD was also associated with an increased risk of urine leakage (р = 0.04). CKD was associated with poor healing of the wound in the renal parenchyma, which could explain the suggestion made by Zargar et al. [11] on its influence on the formation of UFs. The volume of blood loss also increased the risk of urine leakage, which was associated with the complicated technique of renal tumor resection. Surgeons’ experience in PN performed with low-invasive access also influenced the rate of urine leakage regardless of the tumor degree (except for endophytic growth) and application of an intraoperative ureteral catheter. The curve of surgeons’ training showed that the risk of UF development was 7.8 times higher (р = 0.001). When the tumor was close to the PCS, the risk of this complication increased (р = 0.003). It is not surprising because complete dissection of such tumors is impossible without pyelotomy, which in turn, increases the risk of urinal leakage. This was published earlier in major multi-institutional studies [49][50][54]. The data provided by several authors on the risk factors of UFs after PN are presented in Table 3.
Table 3. Preoperative predictors of urinary fistulas after partial nephrectomy
|
Authors, year of publication |
Tumor size |
Tumor endophyticity |
Proximity to the PCS |
Closing the defect of the PCS |
WIT |
Blood loss |
Other factors |
|
Kundu et al., 2010 [13] |
Yes |
Yes |
No |
No |
Yes |
Yes |
No |
|
Wheat et al., 2013 [7] |
Yes |
Yes |
Yes |
No |
No |
No |
No |
|
Zargar et al., 2014 [14] |
No |
No |
Yes |
No |
No |
Yes |
Use of laparoscopy, moderate to severe CKD |
|
Tomaszewski et al., 2014 [30] |
No |
Yes |
No |
Yes |
Yes |
Yes |
Increased operation time, intrarenal pelvis |
|
Potretzke et al., 2016 [15] |
Yes |
No |
Yes |
Yes |
Yes |
No |
Tumor localization in the sinus, time of surgery |
|
Peyton et al., 2020 [53] |
ND |
ND |
ND |
No |
No |
Yes |
Open PN, non-use of sliding-clip renorraphy |
|
Notes: PCS – pelvicalyceal system; WIT – warm ischemia time; CKD – chronic kidney disease; PN – partial nephrectomy; ND – no data. |
|||||||
Bruner et al. [55] studied the association between the RENAL nephrometry score and extravasation of urine after PN in patients with tumors < 7 cm. The authors compared the data received from 31 patients with UFs and 124 patients without UFs. In the first group, the size of the tumor was 3.4 (1.5–5.9) cm, the mean nephrometric score was 9 (5–11). Each point was associated with an increase in the risk of the development of UF by 35% (р = 0.009). The multivariate analysis of tumors (<50% exophytic (р = 0.002), completely endophytic (р = 0.002), and localized in the renal pole (р = 0.017)) showed that they were associated with extravasation of urine. Considering a low rate of UFs and differences in the complexity of tumors in groups of RAPN and LPN, it is hard to conclude this issue. However, it can be suggested that better visualization was provided by a robotic console and video camera, and the articulation capacity of robotic tools contributed to better identification and closing of the PCS defect [56][57]. This can be another explanation of a decrease in the feasibility of the intraoperative ureteral catheter in the cohort that underwent RAPN. Bove et al. [58] concluded that in the selected group of patients (n = 103) with average (< 3 cm) size renal tumor, who were to undergo LPN, ureteral catheter was contraindicated. Zargar et al. [11] confirmed this statement in a larger study (n = 1019) that included patients after LPN and RAPN. In 425 of these patients, the tumor was located proximal to the PCS, and 169 patients had a tumor size > 4 cm.
Potretzke et al. [15] reported 14 symptomatic UFs (0.78%) in 1791 patients after RAPN. The average score by the nephrometry score was 7.2 points in the whole cohort, and 8.0 points in patients with UFs. The tumor size and its localization in the area of the sinus were predictors of this complication (р = 0.021 and 0.025, respectively). UFs were predicted by the necessity to restore PCS (р = 0.018), time of surgery (р = 0.006), and WTI (р = 0.005). Patients with the suturing of the PCS defect and formed UFs had longer surgery time than patients with the closing of the PCS defect and without urine leakage (239.6 versus 189.8 min; р = 0.031). These groups were similar by the size of the tumor, nephrometry score, WTI, the volume of the blood loss, and location in the sinus.
Modern sliding-clip renorraphy allows the surgeon to apply stronger tension to each suture in comparison with a simple surgical knot [59][60]. The application of barbed sutures decreases the risk of WTI that is often referred to as a risk factor for extravasation of urine. Thus, modern suture material can reduce the risk of the development of UFs. In general, urologists note a decrease in the rate of PN complications, including UFs, along with the development and improvement of renorraphy techniques [47]. Tomaszevski et al. [30] studied the influence of the Renal pelvic score on the UFs after PN. A total of 831 patients underwent PN (57.3% RAPN) for low (28.8%), medium (56.5%), and high (14.5%) complexity of tumors and tumor sizes 3.0 ± 2.2 cm. Fifty-four (6.5%) patients developed clinical and radiological signs of urine leakage (mean time of urine leakage was 63 ± 53 days, range from 8 to 230 days) with the mean time of observation 25.5 ± 51.9 months. Thirty patients (55.6%) had expressed urine leakage that required secondary interventions: stenting (40.7%), nephrostome installation (7.4%), percutaneous abdominal drainage (14.8%); and in 5 (9.3%) patients, nephrectomy, reconstruction, or angioembolization was performed. The comparison of patients with urine leakage and without it showed a significant difference in the rate of PCS damage (79.6%/20.4%, р < 0.001), the time of hospitalization, blood loss volume, WTI, and surgical access. The comparison of intrarenal and extrarenal pelvises revealed significant differences in the type of surgery (54.2/41.6 % OPN, р = 0.040), opening of the PCS (52.8/30.0%, р < 0.001), time of surgery (205 ± 77/186 ± 61 min, р = 0.041), WTI (31 ± 19/27 ± 10 min, р = 0.042), and tumor stage. The intrarenal pelvises were identified in 72 (8.7%) of patients that had a high occurrence rate of UFs (43.1/3.0 %, р < 0.001) and significant volume of discharge that required invasive intervention (23.6/1.7 %, р < 0.001) in comparison with patients with extrarenal pelvises but without a prolonged period of urine leakage (65 ± 8/56 ± 9 days, р = 0.098). The multivariate analysis showed that the intrarenal pelvis was the most predictive factor than the tumor endophyticity (p = 0.018) and the opening of the PCS during surgery (p <0.001). According to the authors, along with the validation of the Renal Pelvic Score, preoperative identification of patients with increased risk of urine leakage should be included in the algorithms of perioperative treatment and consulting. Peyton et al. [53] analyzed the results of 975 patients after OPN and RAPN. In 2.3% of them, UFs were diagnosed. In this group, the mean nephrometry score was 8 and the mean time of diagnosis of this complication was 3.5 days. The risk factors associated with urine leakage included open surgery, increased volume of blood loss, and non-use of sliding-clip renorraphy (р < 0.05).
Prevention of urinary fistulas after partial nephrectomy
Intraoperative diagnostics of PCS injury during PN, its timely closing, and checking for leakage can significantly minimize the risks of UF development. The most widespread type of diagnostics for PCS injury is the introduction of methylene blue via a retrograde ureteral catheter. This is a routine manipulation during PN that is used as an intraoperative measure for the minimization of the risk of urine leakage. This practice was common in early OPN, and later surgeons began to use it during LPN and RAPN [20][47][61]. The ureteral catheterization is time-consuming and bears the risks of complications. However, urologists are familiar with it, it is widely available and cheap. The introduction of methylene blue after tumor resection excludes the PCS defect or reveals it for suturing. Still, there is no common opinion on this procedure in the published literature. Zargar et al. [11] studied the results of 1019 low-invasive PNs (567 LPN and 452 RAPN). A decision on the ureteral catheterization for the intraoperative introduction of methylene blue was made individually in each case. Under general anesthesia, in the lithotomy position of the patient, a rigid cystoscope was used for the installation of an external ureteral stent with one pigtail that was attached to the Foley catheter. The intraoperative ureteral catheterization was performed in 528 (51.8%) cases. LPN and RAPN included the identification of tumor under ultrasound control and its demarcation, clamping of the renal artery, tumor dissection, and closing of the renal defect with two layers of horizontal mattress suture, one of which closed the PCS defect and the other brought the edges of the parenchyma closer. The authors performed a regression analysis of such parameters as surgical access, the curve of education (outside or inside), age, BMI, kidney functioning (moderate to severe CKD in comparison with light CKD to normal functioning), size, and endophycity of tumor (> 50% or < 50% endophytic), proximity to the renal cavitary system (>7 or < 7 mm), WTI, blood loss volume, and intraoperative ureteral catheterization (yes or no). In the postoperative period, UFs were observed in 31 patients (3.0%) that underwent upper urinary tract stenting and dynamic computed tomography (CT). The comparison of groups with ureteral catheterization and those without it did not reveal any significant differences in the age and sex of patients, body mass index, kidney functioning, and complexity of the tumor. Tumors in the group of patients with ureteral catheterization had lower general nephrometry scores, longer distances to the PCs, smaller size, and more exophytic localization in comparison with the group of patients without catheterization. The majority of PNs in the group of catheterizations was performed laparoscopically, while in the other group, laparoscopy was performed only to 20% of patients (88.6% versus 20.2%, р = 0.001), the operation time was also long (211 versus 180 minutes, р < 0.01). It was surprising that the rate of UFs was higher in the group of catheterizations than in the group without it (24 (4.6%)/7 (1.4%), р = 0.001). An increase in the experience in LPN did not lead to the differences in the practical application of ureteral catheters. Their coefficient of usefulness was 80.2% for surgeons within the curve of training and 83% for surgeons outside it (р = 0.49). For RAPN, these ratios were 49.4% and 4.7%, respectively (р = 0.001). In comparison with RAPN, tumors resected laparoscopically were less complicated and had lower nephrometry scores, which was also observed in other series of cases [62][63].
Instrumental examination of patients in the short-term postoperative period after PN is not a standard and routine procedure. A common practice among surgeons is the placement of pararenal drainage that is usually removed on Days 1–2 after PN. According to the published data, the earliest symptomatic urine leakage developed on Day 3 after the surgery. In the author’s practice, a gradual increase in the volume of hemorrhagic discharge via the drainage, which became lighter in color, was often observed. The biochemical assay showed the presence of urine in the drainage discharge. For this reason, in patients with a high risk of this complication, the drainage system should not be removed early. It should be left in a patient and monitored for 3–5 days after PN. Routine ultrasound imaging (USI) of kidneys is performed in patients within the first day after the surgery to exclude the extravasation of urine or hematomas. The drainage is removed only in patients with a small volume of discharge (<20–30 ml) and lack of paranephral alterations. However, despite this fact, after discharge from the hospital, some patients were diagnosed with extravasations of urine by the results of USI or CT that required repeated hospitalization and percutaneous drainage of the retroperitoneal space.
Kundu et al. [13] studied the results of 1118 PNs (OPN or LPN) for localized renal cell carcinoma from 1989 to 2007. Before the discharge, the volume of the drainage discharge and the level of creatinine were estimated. If the level of the latter was equal to or lower than the level of creatinine in the blood serum, the drainage was removed before the patient’s discharge from the hospital. On the contrary, if the creatinine level was higher, the drainage was left until the volume of the discharge was reduced. UFs were identified by general complications that developed within 90 days after PN. They were distributed by the Clavien-Dindo system. Patients that were discharged with drainage that was not removed for 14 days after the surgery were considered to have UFs. Otherwise, this complication manifested itself later when patients developed signs and symptoms of paranephral extravasation of urine. UFs were observed in 52 (4.6%) out of 1118 patients. Age, sex, tumor histology, multifocality, and the type of surgery were not statistically associated with an increased risk of UFs. The mean size of the tumor was significantly higher in patients that developed urine leakage in comparison with those that did not have it (3.5 versus 2.6 cm, р = 0.03). In patients with tumors > 2.5 cm, the risk of the development of UFs after PN was two times higher than in patients with tumors < 2.5 cm (6% versus 3%, р = 0.03). The volume of blood loss was significantly higher than in patients with UFs (400 versus 300 ml, р < 0.001), as well as WTI (50 versus 39 minutes, р < 0.001).
Kunitsky et al. [64] showed that intraoperative diagnostics of PCS injury and its adequate closure were technically possible in patients after PN. They described the technique and their experience on the intravenous application of fluorescein that is quickly excreted with urine during PN for the identification of PCS damage. The authors used this method in 48 RAPNs. They evaluated the characteristics of patients, tumors, and the rate of UFs. The average RENAL score was 7.8; the distance from the PCS was 3.3 mm. During RAPN and clamping of the renal artery, 5 ml of fluorescein were injected i.v. right after the resection of the tumor and removing the vascular clamp. In patients who underwent ischemia-free RAPN, the drug was injected right after the removal of the neoplasm. This method allowed surgeons to reveal intraoperative urine leakage from the operative area in three patients. After this procedure, they did not have UFs or urinomas. According to the authors, this method is promising and will allow minimizing the share of UFs in patients with RAPN.
Delto et al. [65] presented their experience of the early removal of the vascular clamp in patients during RAPN to reduce the rate of such complications as pseudoaneurysm and urine leakage. The authors claim that early restoration of the bloodstream provides better control of the damaged arteries and performs adequate renorraphy after the tumor resection, and reduces the total rate of delayed complications with a lower risk of hemotransfusion. The study included the results of 463 RAPNs. The mean operation time and WTI were 186 and 14.7 minutes, respectively. The volume of blood loss was 242 ml. Thirty-day postoperative complications were observed in 14.7% of patients, in 88% of them, the complications were classified as degrees I–II by the Clavien-Dindo scale. UF was diagnosed only in 1 (0.2%) patient that underwent simultaneous PN and pyelolithotomy for staghorn nephrolithiasis. The share of postoperative hemotransfusion was 1.33%. There were no cases of pseudoaneurysm observed. Considering the obtained results, the authors recommend the technique of early vascular clamp removal to reduce the rate of pseudoaneurysm and UFs. Goboy et al. [66] studied the results of OPN in 512 patients. In 75 (14.6%) of them, the drainage was not used. The authors evaluated clinical data and 90-day postoperative complications. In this group, the mean size of the tumor was 2.0 cm and more than 70% of them were malignant. The total rate of complications was 13.3%. In 4 patients (5.3%), complications were associated with the lack of drainage, including stage I (extravasation of urine), stage II (pararenal accumulation), stage III (urinoma that required percutaneous draining), and stage III (urine leakage with the development of sepsis). Still, the authors recommend not to install drainage in the selected group of patients with small neoplasms after PN. A decision on the necessity of drainage should be made intraoperative individually in each case.
Treatment for urinary fistulas after partial nephrectomy
The management of patients with UFs after PN lacks a precise established algorithm of therapeutic manipulations. Some patients can be actively monitored within several days after the surgery. However, a constant volume of the discharge, signs of paranephral extravasation of urine, and fever require more active additional intervention, like retrograde ureteral stenting with the installation of a urethral catheter [10][11]. The drainage of the upper urinary tract simplifies the outflow of the urine from the PCS and decreases the pressure inside it, which contributes to the healing of the PCS defect. However, if stenting is ineffective, such invasive interventions as percutaneous puncture drainage or repeated surgical intervention may be required. The time of upper urinary tract (UUT) drainage can be long enough for the repeated closure of the UF. Meek et al. [11] observed 21 (13.3%) patients with UFs. In 62% of them, extravasation of urine resolved only after a long-term percutaneous draining. Eight patients (38%) required the placement of the second ureteral stent, which was made, on average, 25 days after PN. On average, the stent remained in patients for 53 days. In these patients, retroperitoneal drainage was removed 26 days after the beginning of the urine leakage. Two patients needed several percutaneous and endoscopic procedures to resolve UF with an average period of urine leakage of 163 days. These patients underwent percutaneous puncture of the PCS vis the damaged calyx, bougienage to 30 Sh with the installation of nephrostomy drainage 22 Sh [67]. Only in 6 (27%) patients, the authors revealed the expected cause of UF that led to an increase in the intrarenal pressure. In 2 patients, obstruction of the pelviureteric junction and proximal ureter was revealed; in 2 patients, calyx orifice stenosis; and in 2 patients, benign prostate hyperplasia with expressed infravesical obstruction. Bruner et al. [68] also performed percutaneous treatment for persisting UFs with calyx orifice stenosis after PN and antegrade dilatation with the placement of nephrostomy drainage.
Kundu et al. [13] observed UFs in 52 (4.6%) patients out of 1118 that underwent PN. In 48 patients (4.0%) out of them, urine leakage developed by the drainage that was placed in the wound within >2 weeks after PN. In 4 (0.4%) patients, after the drainage removal, delayed UF developed. In 36 patients (69%) out of 52, this complication resolved after conservative therapy; in 16 cases (31%), repeated interventions were needed. The median time before the resolution of the urine leakage was 64 (29–96) days. The most widespread intervention used in 8 patients (15%) was ureteral stenting. Two patients (4%) had percutaneous drainage, and 1 patient had a urethral catheter installed, stent + drainage, and ureteroscopy was performed. Potretzke et al. [15] observed UFs in 14 patients (0.78%) out of 1791 that underwent RAPN. The mean time and range of symptoms of urine leakage were 13 (3–32) days. Eight out of 14 (57%) patients required hospitalization, while 8 (57%) and 9 (64%) of them had drainage or stent installed. On average, they were removed in 8 (4–13) and 21 (8–83) days, respectively. Thus, the study by Potretzke et al. [15] disputes routine installation of drainages because urine leakage primarily occurs after their removal. Peyton et al. [53] observed 2.3% of cases of UFs in 875 OPN and LPN. Ten (44%) patients underwent conservative therapy; 9 (39%) patients required stenting; 3 (13%) patients underwent percutaneous nephrostomy, and 1 (4%) patient had puncture drainage of pararenal urinoma. In 1 patient, conservative therapy was ineffective, and 45 days after the primary surgery, radical nephrectomy was performed. The mean time to the stent and drainage removal was 40 ± 17 and 24 ± 7 days, respectively. Five patients with symptomatic UFs were repeatedly hospitalized 3.2 ± 1.8 days after.
However, in a certain group of patients, despite retrograde or percutaneous drainage of UUT, UFs did not heal, and the recovery was delayed. In these cases, urologists performed additional endoscopic interventions. The most frequent methods of treatment for persistent UFs included the simultaneous installation of two stents [11][69][70], retrograde or percutaneous application of fibrin and tissue adhesives (Histoacryl) [71][72][73][74][75], and percutaneous cryoablation of the fistulous passage [76]. Alsikafi et al. [69] installed two ureteral stents for the treatment of non-healing UF. A senior patient with a major central tumor of the only functioning kidney underwent PN. The installation of one stent was ineffective, so two stents were installed in the ipsilateral ureter. The upper end of one stent was installed into the upper calyx, and the other – in the lower calyx. Partalidis et al. [70] observed a patient (37-years-old) who developed UF 15 days after PN for a tumor of the lower pole of the left kidney. Retrograde pyelography confirmed a defect in the lower calyx. The patient got installed simultaneously two stents of different diameters (6 Ch regular and 14 Ch endopyelotomic). Three weeks after the surgery, urine leakage stopped and the area of the PCS defect closed, which was verified by CT. The control examination performed 24 months later did not reveal any recurrence of urine leakage.
Fibrin adhesive was used by various authors for the treatment of UFs in single cases. French and Marcovich [72] described a clinical case, wherein UF after PN was treated by retrograde ureteroscopy and injection of fibrin adhesive in the cavity of the lower calyx. Chiu еt al. [74] observed one patient with a solitary kidney and persisting UF. Conservative therapy was ineffective. The authors performed retrograde ureteroscopy with fulguration of the affected calyx and injection of fibrin adhesive. The examination 13 months later showed that the PCS defect resolved. Bradford and Wolf [71] also described a case of fibrin adhesive application in the percutaneous therapy of UF that did not heal for 4 months after OPN. UF was non-susceptible to long-term stenting and bladder drainage, as well as ureteroscopic fulguration. Fibrin adhesive was injected in the renocutaneous fistula under X-ray control, which led to its fast and complete resolution. Selli et al. [73] used n-butyl-2-cyanoacrylate for the treatment of persisting UFs in 5 patients. Three of them had UF revealed after OPN, LPN, and RAPN. In one case, UF was diagnosed after orthotopic cystoplasty, and one patient was diagnosed with bilateral ureterosigmoanastomosis failure. A ureteral catheter with an open-end was used for retrograde injection of the adhesive in the renal calices, while in cases with pelvic fistulas, adhesive was injected percutaneously. Fluoroscopic control showed closure of UF in all the cases. There were no significant postoperative complications observed. However, for the mean 11-month follow-up period, a positive outcome was observed only in 4 (80%) patients. One patient had recurrent UF after PN and underwent nephrectomy. The authors concluded that this adhesive can be used as first-line therapy for persisting UFs. Gorsi et al. [75] used n-butyl-2-cyanoacrylate in a patient aged 51 years old to close UF after RAPN. The adhesive was injected percutaneously. An immediate decrease in the drainage discharge volume was observed. The drainage was removed on day 3 and the patient was discharged from the hospital on day 5 after the surgery.
Ward et al. [76] observed a 34-year-old patient with persisting UF after RAPN. CT control was used for percutaneous cryoablation of the area of urine extravasation, which was revealed by intravenous injection of a contrasting agent. Further examination after ablation showed UF resolution and preservation of the kidney functioning.
Conclusion
Urinary fistulas are rare complications after PN. Their rate of occurrence significantly reduced with a decrease in the size of diagnosed kidney tumors and active application of laparoscopic and RAPN. However, urologists that apply organ-sparing surgical techniques for renal cell carcinoma will sometimes face UFs in their clinical practice. For this reason, they have to be informed about the causes and methods of treatment for this pathology. The author believes that the present review article will be useful for specialists. In the majority of cases, ureteral stenting provides the resolution of urine leakage after PN. In cases of long-term non-healing urinary fistulas, the installation of two stents or percutaneous injection of fibrin adhesive can be the methods of choice.
References
1. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, Giles RH, Hofmann F, Hora M, Kuczyk MA, Kuusk T, Lam TB, Marconi L, Merseburger AS, Powles T, Staehler M, Tahbaz R, Volpe A, Bex A. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol. 2019;75(5):799-810. DOI: 10.1016/j.eururo.2019.02.011
2. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF, Russo P, Uzzo RG; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271-9. DOI: 10.1016/j.juro.2009.07.004
3. Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55-61; discussion 61-2. DOI: 10.1016/j.juro.2008.09.017
4. Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, Shippee ND, Erwin PJ, Costello BA, Chow GK, Leibovich BC. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012;188(1):51-7. DOI: 10.1016/j.juro.2012.03.006
5. Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65(2):372-7. DOI: 10.1016/j.eururo.2013.06.044
6. Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171(1):130-4. DOI: 10.1097/01.ju.0000101281.04634.13
7. Wheat JC, Roberts WW, Hollenbeck BK, Wolf JS Jr, Weizer AZ. Complications of laparoscopic partial nephrectomy. Urol Oncol. 2013;31(1):57-62. DOI: 10.1016/j.urolonc.2010.11.003
8. Gonzalez-Aguirre AJ, Durack JC. Managing Complications Following Nephron-Sparing Procedures for Renal Masses. Tech Vasc Interv Radiol. 2016;19(3):194-202. DOI: 10.1053/j.tvir.2016.06.004
9. Ryan J, MacCraith E, Davis NF, McLornan L. A systematic management algorithm for perioperative complications after robotic assisted partial nephrectomy. Can Urol Assoc J. 2019;13(11):E371-E376. DOI: 10.5489/cuaj.5750
10. Simmons MN, Gill IS. Decreased complications of contemporary laparoscopic partial nephrectomy: use of a standardized reporting system. J Urol. 2007;177(6):2067-73; discussion 2073. DOI: 10.1016/j.juro.2007.01.129
11. Meeks JJ, Zhao LC, Navai N, Perry KT Jr, Nadler RB, Smith ND. Risk factors and management of urine leaks after partial nephrectomy. J Urol. 2008;180(6):2375-8. DOI: 10.1016/j.juro.2008.08.018
12. Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005;174(3):855-8. DOI: 10.1097/01.ju.0000169453.29706.42
13. Kundu SD, Thompson RH, Kallingal GJ, Cambareri G, Russo P. Urinary fistulae after partial nephrectomy. BJU Int. 2010;106(7):1042-4. DOI: 10.1111/j.1464-410X.2010.09230.x
14. Zargar H, Khalifeh A, Autorino R, Akca O, Brandao LF, Laydner H, Krishnan J, Samarasekera D, Haber GP, Stein RJ, Kaouk JH. Urine leak in minimally invasive partial nephrectomy: analysis of risk factors and role of intraoperative ureteral catheterization. Int Braz J Urol. 2014;40(6):763-71. DOI: 10.1590/S1677-5538.IBJU.2014.06.07
15. Potretzke AM, Knight BA, Zargar H, Kaouk JH, Barod R, Rogers CG, Mass A, Stifelman MD, Johnson MH, Allaf ME, Sherburne Figenshau R, Bhayani SB. Urinary fistula after robot-assisted partial nephrectomy: a multicentre analysis of 1 791 patients. BJU Int. 2016;117(1):131-7. DOI: 10.1111/bju.13249
16. Steinbach F, Stockle M, Muller SC, Thuroff JW, Melchior SW, Stein R, Hohenfellner R. Conservative surgery of renal cell tumors in 140 patients: 21 years of experience. J Urol. 1992;148(1):24-9; discussion 29-30. DOI: 10.1016/s0022-5347(17)36499-6
17. Moll V, Becht E, Ziegler M. Kidney preserving surgery in renal cell tumors: indications, techniques and results in 152 patients. J Urol. 1993;150(2 Pt 1):319-23. DOI: 10.1016/s0022-5347(17)35471-x
18. Campbell SC, Novick AC, Streem SB, Klein E, Licht M. Complications of nephron sparing surgery for renal tumors. J Urol. 1994;151(5):1177-80. DOI: 10.1016/s0022-5347(17)35207-2
19. Lerner SE, Hawkins CA, Blute ML, Grabner A, Wollan PC, Eickholt JT, Zincke H. Disease outcome in patients with low stage renal cell carcinoma treated with nephron sparing or radical surgery. J Urol. 1996;155(6):1868-73. PMID: 8618276
20. Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, Thornton J, Sherief MH, Strzempkowski B, Novick AC. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003;170(1):64-8. DOI: 10.1097/01.ju.0000072272.02322.ff
21. Sanchez-Ortiz R, Madsen LT, Swanson DA, Canfield SE, Wood CG. Closed suction or penrose drainage after partial nephrectomy: does it matter? J Urol. 2004;171(1):244-6. DOI: 10.1097/01.ju.0000099940.02698.38
22. Fergany AF, Saad IR, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol. 2006;175(5):1630-3; discussion 1633. DOI: 10.1016/S0022-5347(05)00991-2
23. Pasticier G, Timsit MO, Badet L, De La Torre Abril L, Halila M, Fassi Fehri H, Colombel M, Martin X. Nephronsparing surgery for renal cell carcinoma: detailed analysis of complications over a 15-year period. Eur Urol. 2006;49(3):485-90. DOI: 10.1016/j.eururo.2005.12.049
24. Patard JJ, Pantuck AJ, Crepel M, Lam JS, Bellec L, Albouy B, Lopes D, Bernhard JC, Guil^ F, Lacroix B, De La Taille A, Salomon L, Pfister C, Sou^ M, Tostain J, Ferriere JM, Abbou CC, Colombel M, Belldegrun AS. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007;52(1):148-54. DOI: 10.1016/j.eururo.2007.01.039
25. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, Frank I, Permpongkosol S, Weight CJ, Kaouk JH, Kattan MW, Novick AC. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178(1):41-6. DOI: 10.1016/j.juro.2007.03.038
26. Stroup SP, Palazzi K, Kopp RP, Mehrazin R, Santomauro M, Cohen SA, Patterson AL, L'Esperance JO, Derweesh IH. RENAL nephrometry score is associated with operative approach for partial nephrectomy and urine leak. Urology. 2012;80(1):151-6. DOI: 10.1016/j.urology.2012.04.026
27. Lane BR, Novick AC, Babineau D, Fergany AF, Kaouk JH, Gill IS. Comparison of laparoscopic and open partial nephrectomy for tumor in a solitary kidney. J Urol. 2008;179(3):847-51; discussion 852. DOI: 10.1016/j.juro.2007.10.050
28. Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009;55(5):1171-8. DOI: 10.1016/j.eururo.2009.01.042
29. Redshaw JD, West JM, Stephenson RA, Lowrance WT, Hamilton BD, Southwick AW, Dechet CB. Use of a polytetrafluoroethylene (GORE-TEX) bolster to close the renal parenchymal defect during open partial nephrectomy. Urology. 2014;84(3):707-11. DOI: 10.1016/j.urology.2014.06.004
30. Tomaszewski JJ, Smaldone MC, Cung B, Li T, Mehrazin R, Kutikov A, Canter DJ, Viterbo R, Chen DY, Greenberg RE, Uzzo RG. Internal validation of the renal pelvic score: a novel marker of renal pelvic anatomy that predicts urine leak after partial nephrectomy. Urology. 2014;84(2):351-7. DOI: 10.1016/j.urology.2014.05.001
31. Venkatesh R, Weld K, Ames CD, Figenshau SR, Sundaram CP, Andriole GL, Clayman RV, Landman J. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006;67(6):1169-74; discussion 1174. DOI: 10.1016/j.urology.2006.01.089
32. Permpongkosol S, Link RE, Su LM, Romero FR, Bagga HS, Pavlovich CP, Jarrett TW, Kavoussi LR. Complications of 2,775 urological laparoscopic procedures: 1993 to 2005. J Urol. 2007;177(2):580-5. DOI: 10.1016/j.juro.2006.09.031
33. Breda A, Stepanian SV, Lam JS, Liao JC, Gill IS, Colombo JR, Guazzoni G, Stifelman MD, Perry KT, Celia A, Breda G, Fornara P, Jackman SV, Rosales A, Palou J, Grasso M, Pansadoro V, Disanto V, Porpiglia F, Milani C, Abbou CC, Gaston R, Janetschek G, Soomro NA, De la Rosette JJ, Laguna PM, Schulam PG. Use of haemostatic agents and glues during laparoscopic partial nephrectomy: a multi-institutional survey from the United States and Europe of 1347 cases. Eur Urol. 2007;52(3):798-803. DOI: 10.1016/j.eururo.2007.02.035
34. Celia A, Zeccolini G, Guazzoni G, Pansadoro V, Disanto V, Porpiglia F, Milani C, Abbou C, Gaston R, Janetschek G, Soomroo NA, Fornara P, Breda A, Schulam PG, De la Rosette J, Laguna MP, Palou J, Breda G. Laparoscopic nephron sparing surgery: a multi-institutional European survey of 592 cases. Arch Ital Urol Androl. 2008;80(3):85-91. PMID: 19009862
35. Nadu A, Kleinmann N, Laufer M, Dotan Z, Winkler H, Ramon J. Laparoscopic partial nephrectomy for central tumors: analysis of perioperative outcomes and complications. J Urol. 2009;181(1):42-7; discussion 47. DOI: 10.1016/j.juro.2008.09.014
36. Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, Wang AJ, Stifelman MD. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182(3):866-72. DOI: 10.1016/j.juro.2009.05.037
37. Gill IS, Kamoi K, Aron M, Desai MM. 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010;183(1):34-41. DOI: 10.1016/j.juro.2009.08.114
38. Lifshitz DA, Shikanov SA, Deklaj T, Katz MH, Zorn KC, Eggener SE, Shalhav AL. Laparoscopic partial nephrectomy: a singlecenter evolving experience. Urology. 2010;75(2):282-7. DOI: 10.1016/j.urology.2009.07.1351
39. Wang P, Xia D, Wang S. Multiple factor analysis of urine leaks after retroperitoneal laparoscopic partial nephrectomy. Urol Int. 2011;87(4):411-5. DOI: 10.1159/000331905
40. Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology. 2011;78(4):813-9. DOI: 10.1016/j.urology.2011.04.065
41. Mues AC, Okhunov Z, Haramis G, Landman J. Contemporary experience with laparoscopic partial nephrectomy. J Laparoendosc Adv Surg Tech A. 2011;21(4):323-7. DOI: 10.1089/lap.2010.0411
42. Bylund JR, Clark CJ, Crispen PL, Lagrange CA, Strup SE. Hand-assisted laparoscopic partial nephrectomy without formal collecting system closure: perioperative outcomes in 104 consecutive patients. J Endourol. 2011;25(12):1853-7. DOI: 10.1089/end.2011.0175
43. Rogers CG, Singh A, Blatt AM, Linehan WM, Pinto PA. Robotic partial nephrectomy for complex renal tumors: surgical technique. Eur Urol. 2008;53(3):514-21. DOI: 10.1016/j.eururo.2007.09.047
44. Scoll BJ, Uzzo RG, Chen DY, Boorjian SA, Kutikov A, Manley BJ, Viterbo R. Robot-assisted partial nephrectomy: a large single-institutional experience. Urology. 2010;75(6):1328-34. DOI: 10.1016/j.urology.2009.10.040
45. Benway BM, Bhayani SB, Rogers CG, Porter JR, Buffi NM, Figenshau RS, Mottrie A. Robot-assisted partial nephrectomy: an international experience. Eur Urol. 2010;57(5):815-20. DOI: 10.1016/j.eururo.2010.01.011
46. Spana G, Haber GP, Dulabon LM, Petros F, Rogers CG, Bhayani SB, Stifelman MD, Kaouk JH. Complications after robotic partial nephrectomy at centers of excellence: multi-institutional analysis of 450 cases. J Urol. 2011;186(2):417-21. DOI: 10.1016/j.juro.2011.03.127
47. Kaouk JH, Hillyer SP, Autorino R, Haber GP, Gao T, Altunrende F, Khanna R, Spana G, White MA, Laydner H, Isac W, Stein RJ. 252 robotic partial nephrectomies: evolving renorrhaphy technique and surgical outcomes at a single institution. Urology. 2011;78(6):1338-44. DOI: 10.1016/j.urology.2011.08.007
48. Dulabon LM, Kaouk JH, Haber GP, Berkman DS, Rogers CG, Petros F, Bhayani SB, Stifelman MD. Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur Urol. 2011;59(3):325-30. DOI: 10.1016/j.eururo.2010.11.017
49. Ficarra V, Bhayani S, Porter J, Buffi N, Lee R, Cestari A, Mottrie A. Predictors of warm ischemia time and perioperative complications in a multicenter, international series of robot-assisted partial nephrectomy. Eur Urol. 2012;61(2):395-402. DOI: 10.1016/j.eururo.2011.10.046
50. Tanagho YS, Bhayani SB, Kim EH, Figenshau RS. Renal cryoablation versus robot-assisted partial nephrectomy: Washington University long-term experience. J Endourol. 2013;27(12):1477-86. DOI: 10.1089/end.2013.0192
51. Mathieu R, Verhoest G, Droupy S, de la Taille A, Bruyere F, Doumerc N, Rischmann P, Vaessen C, Roupret M, Bensalah K. Predictive factors of complications after robot-assisted laparoscopic partial nephrectomy: a retrospective multicentre study. BJU Int. 2013;112(4):E283-9. DOI: 10.1111/bju.12222
52. Connor J, Doppalapudi SK, Wajswol E, Ragam R, Press B, Luu T, Koster H, Tamang TL, Ahmed M, Lovallo G, Munver R, Stifelman MD. Postoperative Complications After Robotic Partial Nephrectomy. J Endourol. 2020;34(1):42-47. DOI: 10.1089/end.2019.0434
53. Peyton CC, Hajiran A, Morgan K, Azizi M, Tang D, Chipollini J, Gilbert SM, Poch M, Sexton WJ, Spiess PE. Urinary leak following partial nephrectomy: a contemporary review of 975 cases. Can J Urol. 2020;27(1):10118-10124. PMID: 32065869
54. Tanagho YS, Kaouk JH, Allaf ME, Rogers CG, Stifelman MD, Kaczmarek BF, Hillyer SP, Mullins JK, Chiu Y, Bhayani SB. Perioperative complications of robot-assisted partial nephrectomy: analysis of 886 patients at 5 United States centers. Urology. 2013;81(3):573-9. DOI: 10.1016/j.urology.2012.10.067
55. Bruner B, Breau RH, Lohse CM, Leibovich BC, Blute ML. Renal nephrometry score is associated with urine leak after partial nephrectomy. BJU Int. 2011;108(1):67-72. DOI: 10.1111/j.1464-410X.2010.09837.x
56. Mayer WA, Godoy G, Choi JM, Goh AC, Bian SX, Link RE. Higher RENAL Nephrometry Score is predictive of longer warm ischemia time and collecting system entry during laparoscopic and robotic-assisted partial nephrectomy. Urology. 2012;79(5):1052-6. DOI: 10.1016/j.urology.2012.01.048
57. Faria EF, Caputo PA, Wood CG, Karam JA, Nogueras-Gonzalez GM, Matin SF. Robotic partial nephrectomy shortens warm ischemia time, reducing suturing time kinetics even for an experienced laparoscopic surgeon: a comparative analysis. World J Urol. 2014;32(1):265-71. DOI: 10.1007/s00345-013-1115-2
58. Bove P, Bhayani SB, Rha KH, Allaf ME, Jarrett TW, Kavoussi LR. Necessity of ureteral catheter during laparoscopic partial nephrectomy. J Urol. 2004;172(2):458-60. DOI: 10.1097/01.ju.0000130332.35800.08
59. Benway BM, Cabello JM, Figenshau RS, Bhayani SB. Sliding-clip renorrhaphy provides superior closing tension during robot-assisted partial nephrectomy. J Endourol. 2010;24(4):605-8. DOI: 10.1089/end.2009.0244
60. Sammon J, Petros F, Sukumar S, Bhandari A, Kaul S, Menon M, Rogers C. Barbed suture for renorrhaphy during robot-assisted partial nephrectomy. J Endourol. 2011;25(3):529-33. DOI: 10.1089/end.2010.0455
61. Haber GP, Gill IS. Laparoscopic partial nephrectomy: contemporary technique and outcomes. Eur Urol. 2006;49(4):660-5. DOI: 10.1016/j.eururo.2006.02.001
62. Kaouk JH, Khalifeh A, Hillyer S, Haber GP, Stein RJ, Autorino R. Robot-assisted laparoscopic partial nephrectomy: step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur Urol. 2012;62(3):553-61. DOI: 10.1016/j.eururo.2012.05.021
63. Khalifeh A, Autorino R, Hillyer SP, Laydner H, Eyraud R, Panumatrassamee K, Long JA, Kaouk JH. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: a single surgeon experience. J Urol. 2013;189(4):1236-42. DOI: 10.1016/j.juro.2012.10.021
64. Kunitsky K, Lec PM, Brisbane W, Lenis AT, Chamie K. Sodium Fluorescein for Identification of Intraoperative Urine Leaks During Partial Nephrectomy. Urology. 2020;142:249. DOI: 10.1016/j.urology.2020.04.084
65. Delto JC, Chang P, Hyde S, McAnally K, Crociani C, Wagner AA. Reducing Pseudoaneurysm and Urine Leak After Robotic Partial Nephrectomy: Results Using the Early Unclamping Technique. Urology. 2019;132:130-135. DOI: 10.1016/j.urology.2019.05.042
66. Godoy G, Katz DJ, Adamy A, Jamal JE, Bernstein M, Russo P. Routine drain placement after partial nephrectomy is not always necessary. J Urol. 2011;186(2):411-5. DOI: 10.1016/j.juro.2011.03.151
67. Meeks JJ, Smith ND, Lesani OA, Nadler RB. Percutaneous endoscopic management of persistent urine leak after partial nephrectomy. J Endourol. 2008;22(3):485-8. DOI: 10.1089/end.2007.0281
68. Bruner B, Ashley R, Leibovich B, Blute M, LeRoy A. Case report: percutaneous catheter management of persistent urine leak due to iatrogenic infundibular stenosis after partial nephrectomy. J Endourol. 2009;23(1):37-41. DOI: 10.1089/end.2008.0291
69. Alsikafi NF, Steinberg GD, Gerber GS. Dual stent placement for the treatment of a persistent urine leak after partial nephrectomy. Urology. 2001;57(2):355-7. DOI: 10.1016/s0090-4295(00)01000-1
70. Pardalidis P, Andriopoulos N, Kosmaoglou E, Pardalidis N. Massive Dilation of the Ureter: An Endoscopic Management of Persistent Urinary Leak After Partial Nephrectomy. J Endourol Case Rep. 2017;3(1):186-188. DOI: 10.1089/cren.2017.0102
71. Bradford TJ, Wolf JS Jr. Percutaneous injection of fibrin glue for persistent nephrocutaneous fistula after partial nephrectomy. Urology. 2005;65(4):799. DOI: 10.1016/j.urology.2004.10.079
72. French DB, Marcovich R. Fibrin sealant for retrograde ureteroscopic closure of urine leak after partial nephrectomy. Urology. 2006;67(5):1085.e1-3. DOI: 10.1016/j.urology.2005.11.026
73. Selli C, De Maria M, Manica M, Turri FM, Manassero F. Minimally invasive treatment of urinary fistulas using N-butyl-2-cyanoacrylate: a valid first line option. BMC Urol. 2013;13:55. DOI: 10.1186/1471-2490-13-55
74. Chu W, Chien GW, Finley DS. Novel ureteroscopic technique for treatment of prolonged caliceal leak after partial nephrectomy. J Endourol. 2015;29(4):397-400. DOI: 10.1089/end.2014.0441
75. Gorsi U, Kumar S, Tyagi S, Sharma A. A novel approach to postrobot-assisted nephron-sparing surgery persistent urinary leak - Can we glue? Indian J Urol. 2020;36(1):62-64. DOI: 10.4103/iju.IJU_209_19
76. Ward TJ, Ahmed O, Chung BI, Sze DY, Hwang GL. Percutaneous Cryoablation for Successful Treatment of a Persistent Urine Leak after Robotic-Assisted Partial Nephrectomy. J Vasc Interv Radiol. 2015;26(12):1867-70. DOI: 10.1016/j.jvir.2015.08.029
About the Author
B. G. GulievRussian Federation
Bakhman G. Guliev — M.D., Dr. Sc. (M), Full Prof.; Prof., Dept. of Urology, Mechnikov North-West State Medical University; Head, Urology Centre with Robot-assisted Surgery, St. Petersburg Mariinsky Hospital.
191015, St. Petersburg, 41 Kirochnaya st.; 191014, St. Petersburg, 56 Liteiny ave.
Tel.: +7 (921) 945-34-80
Competing Interests:
The author declares no conflicts of interest.
Review
For citations:
Guliev B.G. Urinary fistulas after partial nephrectomy in renal cell carcinoma. Urology Herald. 2021;9(2):111-124. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-2-111-124













































