Scroll to:
Creation of a training simulator model for practising puncture of the kidney calyceal system under ultrasound control
https://doi.org/10.21886/2308-6424-2021-9-1-22-31
Abstract
Introduction. In the modern world, training on medical simulators is actively used in the training of specialists. To improve the skill of puncture of the cavity system of the kidney, many simulators have been created, from biological ones to virtual reality simulators, but all of them have drawbacks - high cost, short shelf life, inconsistency with reality.
Purpose of the study. To create a simulator model that will be identical in its anatomical and acoustic properties to the kidney and adjacent human tissues, as well as convenient to use and affordable for most universities and clinics.
Materials and methods. The samples of simulators based on glycerin and gelatin were created. A study of the speed of sound in all compositions was carried out, as well as a study of track formation after passing the puncture needle, as well as the ability of the compositions to overgrow (sticking) tracks. The model of the simulator was tested by urologists.
Results. As a result of the tests, it was found that the samples based on gelatin and glycerin are more wear-resistant, the shelf life is longer than that of other samples, and this model is as close as possible in its acoustic properties to human tissues. When testing the simulator, specialists highly appreciated the quality of visualization of both the kidney model itself and the needle during puncture, as well as visualization during repeated punctures.
Conclusion. The simulator developed by us can be used to train young specialists, to assess the practical and theoretical skills of graduates within the framework of accreditation, as well as to continuously improve the qualifications of specialists and when planning surgical intervention for a particular patient.
Keywords
For citations:
Gadzhiev N.K., Mishchenko A.A., Britov V.P., Khrenov A.M., Gorelov D.S., Obidnyak V.M., Grigoriev V.E., Semenyakin I.V., Petrov S.B. Creation of a training simulator model for practising puncture of the kidney calyceal system under ultrasound control. Urology Herald. 2021;9(1):22-31. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-1-22-31
Introduction
In the modern world, ultrasound diagnostic is an integral part of the practical activities of most surgeons. Ultrasound is used not only for the diagnosis of various diseases, but also for performing invasive procedures, such as vascular puncture, tissue biopsy, and access during surgical interventions. In this article, the new model of the simulator for puncture of the renal cavity system under ultrasound control will be demonstrated in detail. Ultrasound-guided puncture is one of the basic skills for a urologist. It is used for various diseases of the genitourinary system, including percutaneous nephrostomy ― the installation of drainage into the renal cavity system in case of kidney stone disease, cancer, ureteral strictures and other conditions that disrupt the outflow of urine from the kidney [1]. At the same time, the puncture of the pelvicalyceal system is the first stage of percutaneous nephrolithotripsy [2]. Training on a live patient, from viewpoint of law and ethics, is a controversial topic. Most of the training process can and should be carried out not with respect to the patient, but the training model. According to the research, practice on simulators reduces the learning curve and helps in planning and preparing for surgery [3]. Nowadays, a urologist has an opportunity to work with an extensive range of training models, which include virtual simulators, animal simulator models, corpses simulator models, non-biological simulator-polymer models. However, each simulator has both advantages and disadvantages. Most of the models presented on the market have such disadvantages as short service life and low wear resistance [4]. Animal organs and existing simulator systems do not reproduce the detailed morphology and physical properties of human organs at the appropriate level [5]. Modern models made of polymer materials are characterized by a high cost (starting from 200 thousand rubles), require special storage conditions (are to be kept in the refrigerator), and also have a short service life (up to 6 months, and with active use-up to 7 days). The study aimed to develop a simulator model that will realistically reproduce the ultrasound picture of the kidney and its cavity system and will be available at a price and operating conditions for almost any clinic and university.
Materials and Methods
The development of the simulator model for the renal cavity system puncture was organized in several stages.
The first stage was the selection of materials for creating the model. Compositions based on gelatin have the most similar acoustic characteristics to the human body since they are a component of many organic tissues. The protein that is part of gelatin is completely denatured, which allows it to be used as a gelatinous material. Glycerin is an organic compound, the simplest representative of triatomic alcohols, a viscous transparent liquid with a sweet taste; it is a non-toxic matter. A two-component silicone was used to imitate the simulator skin. Silicones are widely used due to their special properties — from medical equipment to food packaging.
The second stage was the study of the sound speed in the composition. One of the main characteristics of the ultrasound simulator is the identity of the model echogenicity and the real object [6]. The limited number of polymers makes it difficult to obtain a high-quality response to ultrasound exposure. The most promising materials are based on animal proteins, one of which is gelatin. A cell filled with compositions based on gelatin and glycerin was made to assess the effect of the liquid on the speed of sound conduction in the compositions. After the gelatinization process was completed, the sound velocity was measured in the obtained samples.
Therefore, three samples with different gelatin and water contents were made (Table 1).
Table 1. Gelatin and water content in the samples
|
Sample number |
Content of gelatin, mass. h. |
Gelatin weight, g |
Water weight, g |
|
1 |
15 |
33 |
217 |
|
2 |
20 |
42 |
208 |
|
3 |
25 |
50 |
200 |
It was figured out that the sound speed transmission is affected only by a continuous medium, which is a high-molecular compound (gelatin) (Fig. 1).
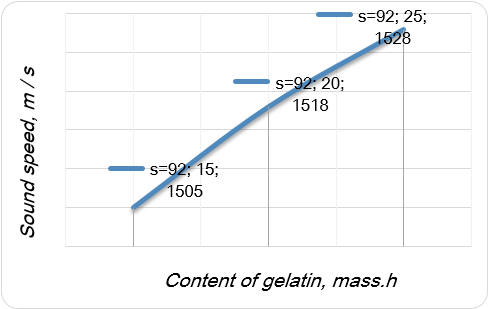
Figure 1. Dependence of the sound speed on the content of gelatin in the composition
It is known that the sound wave speed transmission is affected by a change in the density of the medium. Five samples weighing 250 g with different component contents were made to test this effect (Table 2). The study was conducted at three different temperatures for all the samples (Table 3, Fig. 2).
Table 2. Content of components in samples for the sound speed examination
|
Sample number |
Glycerin / Water Ratio |
Glycerin weight, g |
Water weight, g |
Gelatin weight, g |
|
1 |
100 / 0 |
208 |
0 |
42 |
|
2 |
80 / 20 |
166 |
42 |
42 |
|
3 |
50 / 50 |
104 |
104 |
42 |
|
4 |
20 / 80 |
42 |
166 |
42 |
|
5 |
0 / 100 |
0 |
208 |
42 |
Table 3. Results of the sound speed examination in samples
|
Temperature, °С |
Sound speed (S = 100 mm), m/s |
|
Sample No. 1 |
|
|
23 |
1781 |
|
11 |
1806 |
|
-8.5 |
1847 |
|
Sample No. 2 |
|
|
23 |
1764 |
|
11 |
1793 |
|
-8.5 |
1832 |
|
Sample No. 3 |
|
|
23 |
1761 |
|
11 |
1785 |
|
-8.5 |
1812 |
|
Sample No. 4 |
|
|
23 |
1683 |
|
11 |
1709 |
|
-8.5 |
- |
|
Sample No. 5 |
|
|
23 |
1596 |
|
11 |
1664 |
|
-8.5 |
- |
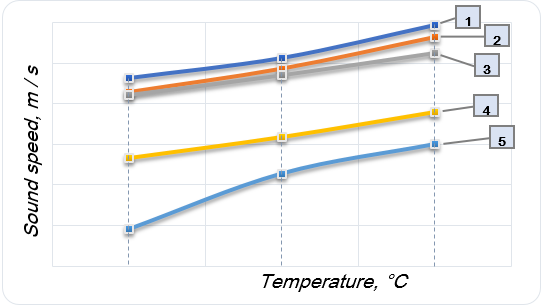
Figure 2. Dependence of the sound speed on the temperature in the studied compositions (box signatures – sample number)
The effect of a significant temperature influence on the change in the density of the medium and, as a result, the sound speed in the material was found. Since the mobility of the polymer chains decreases as the temperature decreases, therefore, the ability to absorb the acoustic wave also decreases, which leads to an increase in the sound speed in the material. As for a composition containing only gelatin and glycerin, this temperature is equal to 12℃. At temperatures below 12℃, medical ultrasound machines interpret the environment as a hyperechoic one, such as bone.
The third stage was to determine the resistance to the formation of tracks. The formation of a puncture course (the so-called “track”) is observed while puncturing simulators made from a gelatin-based composition, after removing the needle from the object. It is not typical for human and animal tissues. The presence of tracks significantly complicates the execution of subsequent punctures (Fig. 3).

Figure 3. “Track” after needle removing
The authors researched the rate of tracks’ “overgrowth” after the puncture for different compositions (Figs. 4, 5). Materials containing only glycerin and compositions with the addition of water were selected as the objects.
It was found out that gelatin-based gel can prolong damage for a certain time if the damage was not critical.
Also, samples were made based on gelatin with the replacement of glycerol with distilled water (from 0% to 80%). Therefore, transparent cells allowing visual control of the speed of closing the puncture mark were filled with the composition.

Figure 4. A sample consisting of gelatin and water, needle track at room temperature immediately after puncture (A) and in 30 minutes (B)

Figure 5. Sample made from gelatin and glycerin, needle track immediately after puncture (А) and in 10 minutes (В)
As a result, the following data was obtained:
- The speed of closing tracks at the initial stage for all compositions is approximately equal.
- Water-containing compositions are prone to loss of moisture due to their evaporation.
As a result, due to the water evaporation, the composition material dries, becomes harder and loses the ability to “overgrow” tracks. It was also found out that the water-based samples dried and cracked after 3 weeks, while no such changes were observed in the glycerol-based samples.
Construction and process design of the medical simulator
According to the basis of the selected composition, a medical simulator was designed for puncture of the human pelvicalyceal kidney system under ultrasound control (Fig. 6, 7).

Figure 6. A model simulating the kidney and the adjacent tissues. The simulator consists of a body (1), a kidney model (5). A collector is mounted in the kidney with an imitation of the pelvicalyceal system (6). The entire structure is located at the bottom of the mold (2), inside the formed body of the simulator made of a composition based on glycerin and gelatin (4), the surface of the “body” is covered with two-component silicone to simulate the skin (3)

Figure 7. Image of the simulator model during the ultrasound examination
Results
The authors have developed a model of a simulator for puncture of the renal cavity system under ultrasound control with realistic anatomical structures, physical and acoustic properties, as close as possible to the natural ones. The polymer base of such a model is a composition of gelatin and glycerin in an anhydrous medium. This made it possible to increase the service life and the wear resistance of the model [6]. The model material has a density similar to one of the human tissues (approximately 10 units, Shore A). The developed simulator can be used for training both students and young medical specialists at the stage of mastering puncture skills.
The created model of the puncture simulator is almost identical to the human kidney cavity system [1]. The simulator allows you to perform more than 300 punctures and has a service life of more than 1 year in the case of being stored at room temperature.
Testing of the created simulator model took place based on the Department of Urology No. 2 of the Pavlov First St. Petersburg State Medical University. Eighteen doctors were offered to fill out questionnaires based on the Likert scale to assess the fitness of the simulator for teaching the puncture skills under ultrasound control (Table 4). The results of this questionnaire are demonstrated in Fig. 8.
Table 4. Questionnaire for doctors
|
Questions |
Excellent |
Good |
Average |
Poor |
Very poor |
|
The visualization quality of kidney model during ultrasound examination |
5 |
4 |
3 |
2 |
1 |
|
The visualization quality of the needle and PCS during puncture |
5 |
4 |
3 |
2 |
1 |
|
The visualization quality during repeated punctures (taking into account the tracks from previous punctures) |
5 |
4 |
3 |
2 |
1 |

Figure 8. Distribution of answers to questions. Question No. 1 ― “The visualization quality of kidney model during ultrasound examination”. Question No. 2 ― “The visualization quality of the needle and PCS during puncture”. Question No. 3 ― “The visualization quality during repeated punctures (taking into account the tracks from previous punctures)”
Experts highly appreciated the quality of visualization of both the kidney layout and the needle during the puncture, as well as visualization during repeated punctures.
Discussion
Puncture of the renal cavity system is an integral part of percutaneous nephrostomy and percutaneous nephrolithotripsy. Many simulators have been developed for this specialists’ skill practicing. For example, pig kidneys covered with tissues imitating the human body tissues were previously used. Such simulators are relatively inexpensive and allow you to work out the skills necessary for percutaneous nephrolithotripsy, such as puncture and augmentation of the puncture course [5]. However, they have various disadvantages, such as short service life and the inability to perform 2 or more manipulations; at the same time, the anatomy of animal kidneys differs from the anatomy of a human organ [7][8]. Virtual reality programs have also been developed for practicing various surgical skills. In particular, PERC Mentor™ (Simbionix; Lod, Israel) is a virtual reality simulator designed specifically for training percutaneous puncture of the renal cavity system [9]. A comparative evaluation of the effectiveness of the VR simulator and practice on live pigs was carried out. The research proved that, despite the high efficiency of these methods, both options are really expensive. In the case of training on live pigs, it is the cost of medicines, veterinarian aid, vivarium presence, etc. On the other hand, it may be compared with the purchase of the PERC Mentor simulator (more than $100,000) taking into account the cost of consumables and maintenance of the simulator (Fig. 9) [10].

Figure 9. PERC Mentor ™ virtual reality simulator (Simbionix; Lod, Israel)
One of the variants of simulators for the pelvicalyceal system puncture is polymer models of kidneys created by using 3D printing. Similar models were developed according to the data of human kidneys computed tomography. Samples of models were made from three different materials – aragose gel, silicone elastomer and polydimethylsiloxane. In the ultrasound study, the aragose gel models demonstrated a better level of visualization. The main advantage of this simulator is the complete anatomical correspondence of the phantom to the human kidney. However, the aragose gel phantom is to be stored at low temperatures. The service life of this phantom is no more than 6 months (according to the observations of specialists from simulation centres), while the simulator offered by the authors can be stored at room temperature for more than 12 months [7].
Non-biological puncture simulators include models created based on a ballistic gel. The latter quite realistically shows the tissues and the course of the needle during ultrasound examination, but the ballistic gel does not have hydrogen bonds, which contribute to the growth of “tracks” formed after the puncture. This feature reduces this simulator service life in comparison with the proposed composition of gelatin [11]. The use of human cadaveric kidneys for training puncture and ultrasound skills has been also described. In the course of this research, it was proved that students successfully mastered the above-mentioned skills after practicing on the simulators. The ultrasound imaging was similar to ultrasound imaging in patients, and it was one of the advantages of this training option. This training method is well suited for medical university students as it allows them to get acquainted with the normal anatomy of the human kidney and adjacent tissues. However, puncture skills training not only in universities but also in hospitals on human corpses is difficult due to the lack of cadaver material and certain storage conditions, as well as the short duration of surgery [12]. The models are useful not only for developing puncture skills and working with ultrasound sensors for clinical residents but also for practicing physicians to maintain the puncture skill at the proper level [13].
Practical intraoperative training continues to remain the main method of teaching percutaneous access under ultrasound guidance. However, training on simulators is an important addition to traditional training [14].
Therefore, practicing skills training on models reduces the learning curve and increases the effectiveness and safety of surgical interventions [3][15].
Conclusion
The simulator developed by the authors can be used for the training of young specialists. In addition, it is possible to use the simulator to assess the practical and theoretical skills of graduates within the framework of the accreditation. The use of this simulator for continuous professional development of specialists and while planning the surgical intervention in a particular patient will increase the effectiveness of the surgery and its safety.
References
1. Dyer RB, Regan JD, Kavanagh P V., Khatod EG, Chen MY, Za-goria RJ. Percutaneous nephrostomy with extensions of the technique: Step by step 1. Radiographics. 2002;22(3):503-25. DOI: 10.1148/radiographics.22.3.g02ma19503
2. Gadzhiev N.K., Britov V.P., Grigor'ev V.E., Mazurenko D.A., Malhasyan V.A., Pisarev A.V., Obidnyak V.M., Tagirov N.S., Popov S.V., Petrov S.B. Creating of the authentic model of human renal collecting system for training percutaneous nephrolithotomy acces in cases of complex kidney stones. Experimental and clinical urology. 2017;2:52-6. (In Russ.). eLIBRARY ID: 29899580
3. Ahmed K, Jawad M, Abboudi M, Gavazzi A, Darzi A, Atha-nasiou T, Vale J, Khan MS, Dasgupta P. Effectiveness of procedural simulation in urology: a systematic review. J Urol. 2011;186(1):26-34. DOI 10.1016/j.juro.2011.02.2684
4. Zaharov D.A., Barysheva O.Ju., Balashov A.T. Zaharov I.D. Vezikova N.N. Fantomy dlja obuchenija navkam ul'trazvukovogo issledovanija, UZI-navigaci, biopsii meto-dom "svobodnojruki". Virtual'nye tehnologii v medicine. 2020;1:49. (In Russ.). DOI: 10.46594/2687-0037_2020_1_49
5. Strohmaier WL, Giese A. Improved ex vivo training model for percutaneous renal surgery. Urol Res. 2009;37(2):107-10. DOI: 10.1007/s00240-009-0180-x
6. Sultan SF, Iohom G, Shorten G. A novel phantom for teaching and learning ultrasound-guided needle manipulation. J Med Ultrasound. 2013;21(3):152-5. DOI: 10.1016/j.jmu.2013.08.001
7. Adams F, Qiu T, Mark A, Fritz B, Kramer L, Schlager D, Wet-terauer U, Miernik A, Fischer P. Soft 3D-Printed Phantom of the Human Kidney with Collecting System. Ann Biomed Eng. 2017;45(4):963-72. DOI: 10.1007/s10439-016-1757-5
8. Stern J, Zeltser IS, Pearle MS. Percutaneous renal access simulators. J Endourol. 2007;21(3):270-3. DOI: 10.1089/end.2007.9981
9. Knudsen BE, Matsumoto ED, Chew BH, Johnson B, Margulis V, Cadeddu JA, Pearle MS, Pautler SE, Denstedt JD. A randomized, controlled, prospective study validating the acquisition of percutaneous renal collecting system access skills using a computer based hybrid virtual reality surgical simulator: phase I. J Urol. 2006;176(5):2173-8. DOI: 10.1016/j.juro.2006.07.011
10. Mishra S, Kurien A, Ganpule A, Muthu V, Sabnis R, Desai M. Percutaneous renal access training: content validation comparison between a live porcine and a virtual reality (VR) simulation model. BJU Int. 2010;106(11):1753-6. DOI: 10.1111/j.1464-410X.2010.09753.x
11. Amini R, Kartchner JZ, Stolz LA, Biffar D, Hamilton AJ, Adhikari S. A novel and inexpensive ballistic gel phantom for ultrasound training. World J Emerg Med. 2015;6(3):225-8. DOI: 10.5847/wjem.j.1920-8642.2015.03.012
12. Meek MEM, Meek JC, Hollowoa B, Li R, Deloney LA, Phelan KD. Lightly Embalmed Cadavers as a Training Tool for Ultrasound-Guided Procedures Commonly Used in Interventional Radiology. Acad Radiol. 2018;25(11):1503-1509. DOI: 10.1016/j.acra.2018.05.019
13. Ristolainen A, Ross P, Gavsin J, Semjonov E, Kruusmaa M. Economically affordable anatomical kidney phantom with calyxes for puncture and drainage training in interventional urology and radiology. Acta Radiol Short Rep. 2014;3(5):2047981614534231. DOI: 10.1177/2047981614534231
14. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-9. DOI: 10.1056/NEJMra054785
15. Hammond L, Ketchum J, Schwartz BF. A new approach to urology training: a laboratory model for percutaneous nephrolithotomy. J Urol. 2004;172(5 Pt 1):1950-2. DOI: 10.1097/01.ju.0000140279.15186.20
About the Authors
N. K. GadzhievRussian Federation
Nariman K. Gadjiev — M.D., Dr.Sc.(M); Head, ESWL and Endovideosurgery Division, Research Center of Urology.
197022, St. Petersburg, 6-8 Lev Tolstoy st.
Competing Interests: no conflicts of interest
A. A. Mishchenko
Russian Federation
Alexandra A. Mishchenko — M.D.; Urologist, ESWL and Endovideosurgery Division, Research Center of Urology.
197022, St. Petersburg, 6-8 Lev Tolstoy st.
Competing Interests: no conflicts of interest
V. P. Britov
Russian Federation
Vladislav P. Britov — Dr.Sc. (Engineering), Full Prof., Head, Dept. of Equipment and Technology of Plastics Processing.
190013, St. Petersburg, 26 Moskovsky ave.
Competing Interests: no conflicts of interest
A. M. Khrenov
Russian Federation
Aleksey M. Khrenov — Senior Lecturer, Dept. of Equipment and Technology of Plastics Processing.
190013, St. Petersburg, 26 Moskovsky ave.
Competing Interests: no conflicts of interest
D. S. Gorelov
Russian Federation
Dmitry S. Gorelov — M.D.; Urologist, ESWL and Endovi-deosurgery Division, Research Center of Urology.
197022, St. Petersburg, 6-8 Lev Tolstoy st.
Competing Interests: no conflicts of interest
V. M. Obidnyak
Russian Federation
Vladimir M. Obidnyak — M.D.; Urologist, ESWL and Endovideosurgery Division, Research Center of Urology.
197022, St. Petersburg, 6-8 Lev Tolstoy st.
Competing Interests: no conflicts of interest
V. E. Grigoriev
Russian Federation
Vladislav E. Grigoriev — M.D.; Urologist, Urology Division.
194044, St. Petersburg, 4/2 Academician Lebedev st.
Competing Interests: no conflicts of interest
I. V. Semenyakin
Russian Federation
Igor V. Semenyakin — M.D., Dr.Sc. (M); Assist., Dept. of Urology.
127473, Moscow, 20, bldg. 1 Delegatskaya st.
Competing Interests: no conflicts of interest
S. B. Petrov
Russian Federation
SergeyB. Petrov — M.D., Dr. Sc. (M); Full Prof.; Head, Research Center of Urology.
197022, St. Petersburg, 6-8 Lev Tolstoy st.
Competing Interests: no conflicts of interest
Review
For citations:
Gadzhiev N.K., Mishchenko A.A., Britov V.P., Khrenov A.M., Gorelov D.S., Obidnyak V.M., Grigoriev V.E., Semenyakin I.V., Petrov S.B. Creation of a training simulator model for practising puncture of the kidney calyceal system under ultrasound control. Urology Herald. 2021;9(1):22-31. (In Russ.) https://doi.org/10.21886/2308-6424-2021-9-1-22-31













































